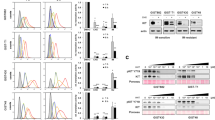
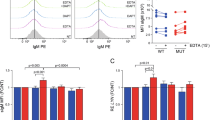
Abstract
Gamma-secretase inhibitors (GSIs) block the activation of the oncogenic protein Notch homolog-1 (NOTCH1) in T cell acute lymphoblastic leukemia (T-ALL). However, limited antileukemic cytotoxicity and severe gastrointestinal toxicity have restricted the clinical application of these targeted drugs. Here we show that combination therapy with GSIs plus glucocorticoids can improve the antileukemic effects of GSIs and reduce their gut toxicity in vivo. Inhibition of NOTCH1 signaling in glucocorticoid-resistant T-ALL restored glucocorticoid receptor autoupregulation and induced apoptotic cell death through induction of the gene encoding BCL-2–like apoptosis initiator-11 (BCL2L11). GSI treatment resulted in cell cycle arrest and accumulation of goblet cells in the gut mediated by upregulation of the gene encoding the transcription factor Krüppel-like factor-4 (Klf4), a negative regulator of the cell cycle required for goblet cell differentiation. In contrast, glucocorticoid treatment induced transcriptional upregulation of cyclin D2 (Ccnd2) and protected mice from developing the intestinal goblet cell metaplasia typically induced by inhibition of NOTCH signaling with GSIs. These results support a role for glucocorticoids plus GSIs in the treatment of glucocorticoid-resistant T-ALL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Evin, G., Sernee, M.F. & Masters, C.L. Inhibition of γ-secretase as a therapeutic intervention for Alzheimer's disease: prospects, limitations and strategies. CNS Drugs 20, 351–372 (2006).
Selkoe, D. & Kopan, R. Notch and presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci. 26, 565–597 (2003).
Weng, A.P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004).
Roy, M., Pear, W.S. & Aster, J.C. The multifaceted role of Notch in cancer. Curr. Opin. Genet. Dev. 17, 52–59 (2007).
Deangelo, D. et al. A phase I clinical trial of the notch inhibitor MK-0752 in patients with T cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias. J. of Clin. Oncol. 2006 ASCO Annual Meeting Proceedings Part I. 24, 6585 (2006).
Palomero, T. et al. CUTLL1, a novel human T-cell lymphoma cell line with t(7;9) rearrangement, aberrant NOTCH1 activation and high sensitivity to γ-secretase inhibitors. Leukemia 20, 1279–1287 (2006).
Lewis, H.D. et al. Apoptosis in T cell acute lymphoblastic leukemia cells after cell cycle arrest induced by pharmacological inhibition of notch signaling. Chem. Biol. 14, 209–219 (2007).
Milano, J. et al. Modulation of Notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82, 341–358 (2004).
van Es, J.H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005).
Wong, G.T. et al. Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem. 279, 12876–12882 (2004).
Searfoss, G.H. et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J. Biol. Chem. 278, 46107–46116 (2003).
Pear, W.S. & Radtke, F. Notch signaling in lymphopoiesis. Semin. Immunol. 15, 69–79 (2003).
Ciofani, M. & Zuniga-Pflucker, J.C. Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 6, 881–888 (2005).
Deftos, M.L., He, Y.W., Ojala, E.W. & Bevan, M.J. Correlating Notch signaling with thymocyte maturation. Immunity 9, 777–786 (1998).
Aster, J.C., Pear, W.S. & Blacklow, S.C. Notch signaling in leukemia. Annu. Rev. Pathol. 3, 587–613 (2008).
Palomero, T., Dominguez, M. & Ferrando, A.A. The role of the PTEN/AKT pathway in NOTCH1-induced leukemia. Cell Cycle 7, 965–970 (2008).
Palomero, T. & Ferrando, A. Oncogenic NOTCH1 control of MYC and PI3K: challenges and opportunities for anti-NOTCH1 therapy in T cell acute lymphoblastic leukemias and lymphomas. Clin. Cancer Res. 14, 5314–5317 (2008).
Seiffert, D. et al. Presenilin-1 and -2 are molecular targets for γ-secretase inhibitors. J. Biol. Chem. 275, 34086–34091 (2000).
Palomero, T. et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc. Natl. Acad. Sci. USA 103, 18261–18266 (2006).
Palomero, T. et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat. Med. 13, 1203–1210 (2007).
Eisen, L.P., Elsasser, M.S. & Harmon, J.M. Positive regulation of the glucocorticoid receptor in human T cells sensitive to the cytolytic effects of glucocorticoids. J. Biol. Chem. 263, 12044–12048 (1988).
Ramdas, J., Liu, W. & Harmon, J.M. Glucocorticoid-induced cell death requires autoinduction of glucocorticoid receptor expression in human leukemic T cells. Cancer Res. 59, 1378–1385 (1999).
Levine, E.G., Peterson, B.A., Smith, K.A., Hurd, D.D. & Bloomfield, C.D. Glucocorticoid receptors in chronic lymphocytic leukemia. Leuk. Res. 9, 993–999 (1985).
Leventhal, B.G. Glucocorticoid receptors in lymphoid tumors. Cancer Res. 41, 4861–4862 (1981).
Pedersen, K.B. & Vedeckis, W.V. Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry 42, 10978–10990 (2003).
Pedersen, K.B., Geng, C.D. & Vedeckis, W.V. Three mechanisms are involved in glucocorticoid receptor autoregulation in a human T lymphoblast cell line. Biochemistry 43, 10851–10858 (2004).
Renner, K., Ausserlechner, M.J. & Kofler, R. A conceptual view on glucocorticoid-lnduced apoptosis, cell cycle arrest and glucocorticoid resistance in lymphoblastic leukemia. Curr. Mol. Med. 3, 707–717 (2003).
Geley, S. et al. Resistance to glucocorticoid-induced apoptosis in human T cell acute lymphoblastic leukemia CEM-C1 cells is due to insufficient glucocorticoid receptor expression. Cancer Res. 56, 5033–5038 (1996).
Schmidt, S. et al. Glucocorticoid resistance in two key models of acute lymphoblastic leukemia occurs at the level of the glucocorticoid receptor. FASEB J. 20, 2600–2602 (2006).
Breslin, M.B., Geng, C.D. & Vedeckis, W.V. Multiple promoters exist in the human GR gene, one of which is activated by glucocorticoids. Mol. Endocrinol. 15, 1381–1395 (2001).
Geng, C.D. & Vedeckis, W.V. c-Myb and members of the c-Ets family of transcription factors act as molecular switches to mediate opposite steroid regulation of the human glucocorticoid receptor 1A promoter. J. Biol. Chem. 280, 43264–43271 (2005).
Nunez, B.S. & Vedeckis, W.V. Characterization of promoter 1B in the human glucocorticoid receptor gene. Mol. Cell. Endocrinol. 189, 191–199 (2002).
Erlacher, M. et al. BH3-only proteins Puma and Bim are rate-limiting for γ-radiation– and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 106, 4131–4138 (2005).
Abrams, M.T., Robertson, N.M., Yoon, K. & Wickstrom, E. Inhibition of glucocorticoid-induced apoptosis by targeting the major splice variants of BIM mRNA with small interfering RNA and short hairpin RNA. J. Biol. Chem. 279, 55809–55817 (2004).
Wang, Z., Malone, M.H., He, H., McColl, K.S. & Distelhorst, C.W. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J. Biol. Chem. 278, 23861–23867 (2003).
Puthalakath, H. et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293, 1829–1832 (2001).
Guo, K. et al. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology 148, 3987–3997 (2007).
Katz, J.P. et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 129, 2619–2628 (2002).
Wei, D., Kanai, M., Huang, S. & Xie, K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis 27, 23–31 (2006).
Jensen, J. et al. Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, 36–44 (2000).
Stappenbeck, T.S., Wong, M.H., Saam, J.R., Mysorekar, I.U. & Gordon, J.I. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr. Opin. Cell Biol. 10, 702–709 (1998).
Sicinski, P. et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384, 470–474 (1996).
Wei, G. et al. Gene expression–based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 10, 331–342 (2006).
Jang, J. et al. Notch1 confers thymocytes a resistance to GC-induced apoptosis through Deltex1 by blocking the recruitment of p300 to the SRG3 promoter. Cell Death Differ. 13, 1495–1505 (2006).
Choi, Y.I. et al. Notch1 confers a resistance to glucocorticoid-induced apoptosis on developing thymocytes by down-regulating SRG3 expression. Proc. Natl. Acad. Sci. USA 98, 10267–10272 (2001).
Jeon, S.H. et al. A new mouse gene, SRG3, related to the SWI3 of Saccharomyces cerevisiae, is required for apoptosis induced by glucocorticoids in a thymoma cell line. J. Exp. Med. 185, 1827–1836 (1997).
Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 (2002).
Acknowledgements
We thank U. Klein for help with flow cytometry analysis; T. Matos for the processing of histological, histochemical and imunohistochemical assays; P. Sicinski (Dana-Farber Cancer Institute) for the Ccnd2-knockout mouse; T. Honjo (Kyoto University) and B. Reitzis (Columbia University) for the Rbpjflox/flox conditional knockout mouse; T. Ludwig (Columbia University) for the Cre-Tam mouse line; A. Kung (Dana-Farber Cancer Institute) for the FUW-Luc neo vector; J. Aster (Brigham and Women's Hospital) for the MigR1 and MigR1-ICN1 retroviral constructs and for the NOTCH1-TAD antibody; W. Hahn (Dana-Farber Cancer Institute) for the pLKO shRNA vector; R. Dalla Favera (Columbia University) for the pCM8-cMYB vector; M.J. Garabedian (New York University) for the pCMV-HA-hGR vector; and N. Wu and C. Wa for pharmacokinetic and drug metabolism analysis. This work was supported by the US National Institutes of Health (R01CA120196 and R01CA129382 to A.F. and R56AI070310, 1R01CA105129 and 1R01CA133379 to I.A.); the Wipe Out Leukemia Forever Foundation (A.F.), the Leukemia and Lymphoma Society (grants 1287-08 and 6237-08 to A.F.), the Charlotte Geyer Foundation (A.F.), the Cancer Research Institute (A.F.), the Swim Across America Foundation (A.F.), the Golfers Against Cancer Foundation (A.F.), the American Cancer Society (RSG0806801 to I.A.), the Città della Speranza Foundation, a Cassa di Risparmio di Padova e Rovigo research grant, a Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale, and the Fondo de Investigacion Sanitaria (grant CD07/00033 to P.J.R.). A.F. and I.A. are Leukemia & Lymphoma Society Scholars. T.P. is a recipient of a Young Investigator Award from the Alex's Lemonade Stand Foundation. I.H. is supported by the Dutch Cancer Society.
Author information
Authors and Affiliations
Contributions
A.F. supervised the project. P.J.R., V.T., T.P., M.L.S, K.B., C.S. and I.H. designed and performed experiments. M.C. performed histological and immunohistochemical studies. E.H. and E.d.S. assisted in bioimaging analysis of tumor xenografts. G.B. provided clinical samples. J.M. provided clinical samples and supervised drug sensitivity assays in these cells. A.F. and I.A. supervised the analysis of Ccnd2−/− mice. C.C.-C. supervised histological and immunohistochemical studies. W.A. designed and performed experiments on the regulation of Klf4 by NOTCH1 and HES1. A.F. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Results and Discussion, Supplementary Methods and Supplementary Figs. 1–17 (PDF 2047 kb)
Rights and permissions
About this article
Cite this article
Real, P., Tosello, V., Palomero, T. et al. γ-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med 15, 50–58 (2009). https://doi.org/10.1038/nm.1900
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1900
This article is cited by
-
Affinity-matured DLL4 ligands as broad-spectrum modulators of Notch signaling
Nature Chemical Biology (2023)
-
Developing Targeted Therapies for T Cell Acute Lymphoblastic Leukemia/Lymphoma
Current Hematologic Malignancy Reports (2023)
-
Interactions of melatonin with various signaling pathways: implications for cancer therapy
Cancer Cell International (2022)
-
A phase 1b study of crenigacestat (LY3039478) in combination with gemcitabine and cisplatin or gemcitabine and carboplatin in patients with advanced or metastatic solid tumors
Cancer Chemotherapy and Pharmacology (2022)
-
Advances of target therapy on NOTCH1 signaling pathway in T-cell acute lymphoblastic leukemia
Experimental Hematology & Oncology (2020)