Abstract
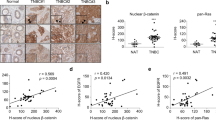
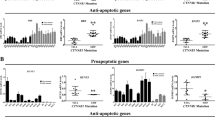
The Wnt–β-catenin and PI3K-AKT-FOXO3a pathways have a central role in cancer. AKT phosporylates FOXO3a, relocating it from the cell nucleus to the cytoplasm, an effect that is reversed by PI3K and AKT inhibitors. Simultaneous hyperactivation of the Wnt–β-catenin pathway and inhibition of PI3K-AKT signaling promote nuclear accumulation of β-catenin and FOXO3a, respectively, promoting cell scattering and metastasis by regulating a defined set of target genes. Indeed, the anti-tumoral AKT inhibitor API-2 promotes nuclear FOXO3a accumulation and metastasis of cells with high nuclear β-catenin content. Nuclear β-catenin confers resistance to the FOXO3a-mediated apoptosis induced by PI3K and AKT inhibitors in patient-derived primary cultures and in corresponding xenograft tumors in mice. This resistance is reversed by XAV-939, an inhibitor of Wnt–β-catenin signaling. In the presence of high nuclear β-catenin content, activation of FOXO3a by PI3K or AKT inhibitors makes it behave as a metastasis inductor rather than a proapoptotic tumor suppressor. We show that it is possible to evaluate the β-catenin status of patients' carcinomas and the response of patient-derived cells to target-directed drugs that accumulate FOXO3a in the nucleus before deciding on a course of treatment. We propose that this evaluation could be essential to the provision of a safer and more effective personalized treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Jänne, P.A. & Mayer, R.J. Chemoprevention of colorectal cancer. N. Engl. J. Med. 342, 1960–1968 (2000).
Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 (2006).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Pálmer, H.G. et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J. Cell Biol. 154, 369–387 (2001).
Olson, L.E. et al. Homeodomain-mediated β-catenin-dependent switching events dictate cell-lineage determination. Cell 125, 593–605 (2006).
Pálmer, H.G., Anjos-Afonso, F., Carmeliet, G., Takeda, H. & Watt, F.M. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE 3, e1483 (2008).
Essers, M.A. et al. Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science 308, 1181–1184 (2005).
Calnan, D.R. & Brunet, A. The FoxO code. Oncogene 27, 2276–2288 (2008).
van der Horst, A. & Burgering, B.M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 8, 440–450 (2007).
Myatt, S.S. & Lam, E.W. The emerging roles of forkhead box (Fox) proteins in cancer. Nat. Rev. Cancer 7, 847–859 (2007).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Dehner, M., Hadjihannas, M., Weiske, J., Huber, O. & Behrens, J. Wnt signaling inhibits Forkhead box O3a–induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J. Biol. Chem. 283, 19201–19210 (2008).
Walther, A. et al. Genetic prognostic and predictive markers in colorectal cancer. Nat. Rev. Cancer 9, 489–499 (2009).
Dienstmann, R., Rodon, J., Markman, B. & Tabernero, J. Recent developments in anti-cancer agents targeting PI3K, Akt and mTORC1/2. Recent Pat. Anticancer. Drug Discov. 6, 210–236 (2011).
Yang, L. et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 64, 4394–4399 (2004).
Zheng, Y. et al. Novel phosphatidylinositol 3-kinase inhibitor NVP-BKM120 induces apoptosis in myeloma cells and shows synergistic anti-myeloma activity with dexamethasone. J Mol Med (Berl) (2011).
Liu, P., Cheng, H., Roberts, T.M. & Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 8, 627–644 (2009).
Hoffman, K. et al. Phase I–II study: triciribine (tricyclic nucleoside phosphate) for metastatic breast cancer. Cancer Chemother. Pharmacol. 37, 254–258 (1996).
Bendell, J.C. et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 30, 282–290 (2012).
Dijkers, P.F., Medema, R.H., Lammers, J.W., Koenderman, L. & Coffer, P.J. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10, 1201–1204 (2000).
Seoane, J., Le, H.V., Shen, L., Anderson, S.A. & Massague, J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117, 211–223 (2004).
Hoogeboom, D. et al. Interaction of FOXO with β-catenin inhibits β-catenin/T cell factor activity. J. Biol. Chem. 283, 9224–9230 (2008).
Kops, G.J. et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321 (2002).
Brabletz, T. et al. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 98, 10356–10361 (2001).
Delpuech, O. et al. Induction of Mxi1-SR α by FOXO3a contributes to repression of Myc-dependent gene expression. Mol. Cell. Biol. 27, 4917–4930 (2007).
Wells, C.D. et al. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125, 535–548 (2006).
Yamashiro, S., Abe, H. & Mabuchi, I. IQGAP2 is required for the cadherin-mediated cell-to-cell adhesion in Xenopus laevis embryos. Dev. Biol. 308, 485–493 (2007).
Natale, D.R. & Watson, A.J. Rac-1 and IQGAP are potential regulators of E-cadherin-catenin interactions during murine preimplantation development. Gene Expr. Patterns 2, 17–22 (2002).
Tsai, M.S., Hornby, A.E., Lakins, J. & Lupu, R. Expression and function of CYR61, an angiogenic factor, in breast cancer cell lines and tumor biopsies. Cancer Res. 60, 5603–5607 (2000).
Dhawan, P. et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Invest. 115, 1765–1776 (2005).
Huang, S.M. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 (2009).
Cross, D.A., Alessi, D.R., Cohen, P., Andjelkovich, M. & Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995).
Brabletz, T., Jung, A., Dag, S., Hlubek, F. & Kirchner, T. β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 155, 1033–1038 (1999).
Samuels, Y. et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7, 561–573 (2005).
Yoeli-Lerner, M. et al. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell 20, 539–550 (2005).
Chen, J. et al. Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS ONE 5, e12293 (2010).
Storz, P., Doppler, H., Copland, J.A., Simpson, K.J. & Toker, A. FOXO3a promotes tumor cell invasion through the induction of matrix metalloproteinases. Mol. Cell. Biol. 29, 4906–4917 (2009).
Paik, J.H. et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 (2007).
Jun, T., Gjoerup, O. & Roberts, T.M. Tangled webs: evidence of cross-talk between c-Raf-1 and Akt. Sci. STKE 1999, PE1 (1999).
Zunder, E.R., Knight, Z.A., Houseman, B.T., Apsel, B. & Shokat, K.M. Discovery of drug-resistant and drug-sensitizing mutations in the oncogenic PI3K isoform p110 α. Cancer Cell 14, 180–192 (2008).
Gomes, A.R., Brosens, J.J. & Lam, E.W. Resist or die: FOXO transcription factors determine the cellular response to chemotherapy. Cell Cycle 7, 3133–3136 (2008).
Obexer, P., Geiger, K., Ambros, P.F., Meister, B. & Ausserlechner, M.J. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 14, 534–547 (2007).
Rochat-Steiner, V. et al. FIST/HIPK3: a Fas/FADD-interacting serine/threonine kinase that induces FADD phosphorylation and inhibits fas-mediated Jun NH(2)-terminal kinase activation. J. Exp. Med. 192, 1165–1174 (2000).
Tourneur, L. & Chiocchia, G. FADD: a regulator of life and death. Trends Immunol. 31, 260–269 (2010).
Shackleton, M., Quintana, E., Fearon, E.R. & Morrison, S.J. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 138, 822–829 (2009).
Fodde, R. & Brabletz, T. Wnt/β-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 19, 150–158 (2007).
Clevers, H. The cancer stem cell: premises, promises and challenges. Nat. Med. 17, 313–319 (2011).
Vermeulen, L. et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476 (2010).
Brabletz, T., Jung, A., Spaderna, S., Hlubek, F. & Kirchner, T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer 5, 744–749 (2005).
Todaro, M. et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 1, 389–402 (2007).
Van der Flier, L.G. et al. The intestinal Wnt/TCF signature. Gastroenterology 132, 628–632 (2007).
Kreso, A. & O'Brien, C.A. Colon cancer stem cells. Curr. Protoc. Stem Cell Biol. 7, 3.1.1–3.1.12 (2008).
Céspedes, M.V. et al. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am. J. Pathol. 170, 1077–1085 (2007).
Ueno, H., Murphy, J., Jass, J.R., Mochizuki, H. & Talbot, I.C. Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40, 127–132 (2002).
Barbáchano, A. et al. SPROUTY-2 and E-cadherin regulate reciprocally and dictate colon cancer cell tumourigenicity. Oncogene 29, 4800–4813 (2010).
Arques, O., Chicote, I., Tenbaum, S., Puig, I. & Palmer, H.G. Standardized relative quantification of immunofluorescence tissue staining. Protoc. Exchange published online, doi:10.1038/protex.2012.008 (2 April 2012).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002).
Aguilar, S. et al. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE 4, e8013 (2009).
Barde, I. et al. Efficient control of gene expression in the hematopoietic system using a single Tet-on inducible lentiviral vector. Mol. Ther. 13, 382–390 (2006).
Fuerer, C. & Nusse, R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLoS ONE 5, e9370 (2010).
Acknowledgements
We thank W. Shao (Novartis Institutes for BioMedical Research, Inc.) for providing the experimental drug, XAV-939, and its inactive analog, LDW-643, and we thank K.F. Becker (Technische Universität München, Germany) for providing the antibody to Snail1. We also thank P.J. Coffer (Utrecht, Netherlands) for providing the pcDNA3-FOXO3a(A3):ERTM expression plasmid, as well as H. Clevers (Utrecht, Netherlands) for providing L8 colon cancer cells. J. Seoane and G. Folch (VHIO, Barcelona, Spain) provided technical advice and reagents. We thank R. Luthra (Molecular Diagnostics Laboratory, MD Anderson Cancer Center, Houston, Texas, USA) for providing the CLIA panel of somatic mutations. We thank M.J. Larriba (Instituto de Investigaciones Biomédicas 'Alberto Sols', Consejo Superior de Investigaciones Científicas-Universidad Autónoma de Madrid, Madrid, Spain) for providing the reagents necessary for the analysis of Zeb1 and Slug expression and M. Scaltriti (Massachusetts General Hospital Cancer Center, Harvard Medical School, Charlestown, Massachusetts, USA) for supplying the lentiviruses used to knock down IQGAP2 expression. We acknowledge R. Rycroft and A. Wren for their valuable assistance in the preparation of the English version of the manuscript. Experiments were supported by a VHIO starting grant and grants from Fondo de Investigaciones Sanitarias–Instituto de Salud Carlos III (ISCIII) (FIS-PI081356, RETICC-RD06/0020/0075 and RETICC-RD06/0020/0009), and Plan Nacional de Biomedicina, Ministerio de Ciencia e Innovación (SAF-18302). S.P.T. was supported by a Fundació Olga Torres Fellowship, I.P. was funded by the Fundación Científica de la Asociación Española Contra el Cancer (AECC), and H.G.P. was supported by the Miguel Servet Program, ISCIII.
Author information
Authors and Affiliations
Contributions
S.P.T., P.O.-M. and I.P. contributed equally to the experiments. S.P.T. also contributed to writing the manuscript, and I.C. also performed experiments. O.A. helped to perform IQGAP2 knockdown experiments. S.L. classified human tumors according to histopathological criteria and built the tissue arrays. Y.F. performed and analyzed live imaging assays (IVIS), which were supervised by S.S. J.R.H., S.R. and J.D.G. participated in live imaging experiments. L.M. and A.V. performed mutational analysis of human colon carcinomas. S.A. cloned and tested lentiviral constructs. S.R.y.C. provided human specimens. E.E. performed surgery on patients with colon cancer. J.B. supervised the project. J.T. supervised the project and provided clinical follow up on all the patients included in the study. A.M. and H.G.P. wrote the manuscript and supervised the project. H.G.P. was responsible for designing all the experiments and analyzing and interpreting all data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–21 and Supplementary Tables 1–9 (PDF 4296 kb)
Rights and permissions
About this article
Cite this article
Tenbaum, S., Ordóñez-Morán, P., Puig, I. et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med 18, 892–901 (2012). https://doi.org/10.1038/nm.2772
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2772
This article is cited by
-
APC/PIK3CA mutations and β-catenin status predict tankyrase inhibitor sensitivity of patient-derived colorectal cancer cells
British Journal of Cancer (2024)
-
FOXO3a-interacting proteins’ involvement in cancer: a review
Molecular Biology Reports (2024)
-
Enhanced anticancer synergy of LOM612 in combination with selinexor: FOXO1 nuclear translocation-mediated inhibition of Wnt/β-catenin signaling pathway in breast cancer
Cancer Chemotherapy and Pharmacology (2024)
-
Tankyrase inhibition interferes with junction remodeling, induces leakiness, and disturbs YAP1/TAZ signaling in the endothelium
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Serpina3n/serpina3 alleviates cyclophosphamide-induced interstitial cystitis by activating the Wnt/β-catenin signal
International Urology and Nephrology (2023)