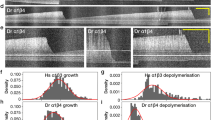
Abstract
Bundles of taxol-stabilized microtubules (MTs)—hollow tubules comprised of assembled αβ-tubulin heterodimers—spontaneously assemble above a critical concentration of tetravalent spermine and are stable over long times at room temperature. Here we report that at concentrations of spermine several-fold higher the MT bundles (BMT) quickly become unstable and undergo a shape transformation to bundles of inverted tubulin tubules (BITT), the outside surface of which corresponds to the inner surface of the BMT tubules. Using transmission electron microscopy and synchrotron small-angle X-ray scattering, we quantitatively determined both the nature of the BMT-to-BITT transformation pathway, which results from a spermine-triggered conformation switch from straight to curved in the constituent taxol-stabilized tubulin oligomers, and the structure of the BITT phase, which is formed of tubules of helical tubulin oligomers. Inverted tubulin tubules provide a platform for studies requiring exposure and availability of the inside, luminal surface of MTs to MT-targeted drugs and MT-associated proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bray, D. Cell Movements: From Molecules to Motility 2nd edn (Garland, 2001).
Thomas, T. D., Earnshaw, W. C. & Lippincott-Schwartz, J. Cell Biology 2nd edn (Saunders, Elsevier, 2008).
Israelachvili, J. N. Intermolecular & Surface Forces (Academic, 1992).
Sackmann, E. Membrane bending energy concept of vesicle-shape and cell-shape and shape-transitions. FEBS Lett. 346, 3–16 (1994).
Chiruvolu, S. et al. A phase of liposomes with entangled tubular vesicles. Science 266, 1222–1225 (1994).
Zidovska, A. et al. Block liposomes from curvature-stabilizing lipids: Connected nanotubes, -rods and -spheres. Langmuir 25, 2979–2985 (2009).
Zidovska, A. et al. Block liposome and nanotube formation is a general phenomenon of membranes containing multivalent lipids. Soft Matter 7, 8363–8369 (2011).
Seeman, N. C. & Belcher, A. M. Emulating biology: Building nanostructures from the bottom up. Proc. Natl Acad. Sci. USA 99, 6451–6455 (2002).
Ringler, P. & Schulz, G. E. Self-assembly of proteins into designed networks. Science 302, 106–109 (2003).
Seeman, N. C. DNA in a material world. Nature 421, 427–431 (2003).
Chworos, A. et al. Building programmable jigsaw puzzles with RNA. Science 306, 2068–2072 (2004).
Nogales, E., Wang, H. W. & Niederstrasser, H. Tubulin rings: Which way do they curve. Curr. Opin. Struct. Biol. 13, 256–261 (2003).
Lowe, J., Li, H., Downing, K. H. & Nogales, E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 313, 1045–1057 (2001).
Ravelli, R. B. G. et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428, 198–202 (2004).
Mandelkow, E. M., Mandelkow, E. & Milligan, R. A. Microtubule dynamics and microtubule caps: A time-resolved cryo-electron microscopy study. J. Cell Biol. 114, 977–991 (1991).
Nogales, E., Wolf, S. G., Khan, I. A., Luduena, R. F. & Downing, K. H. Structure of tubulin at 6.5 Å and location of the taxol-binding site. Nature 375, 424–427 (1995).
Mitra, A. & Sept, D. Taxol allosterically alters the dynamics of the tubulin dimer and increases the flexibility of microtubules. Biophys. J. 95, 3252–3258 (2008).
Jordan, M. A. & Wilson, L. Microtubules as a target for anticancer drugs. Nature Rev. Cancer 4, 253–265 (2004).
Jordan, M. A. & Wilson, L. in Cancer Drug Discovery and Development, The Role of Microtubules in Cell Biology, Neurobiology, and Oncology (ed. Fojo, T.) 47–81 (Humana Press, 2008).
Elie-Caille, C. et al. Straight GDP-tubulin protofilaments form in the presence of taxol. Curr. Biol. 17, 1765–1770 (2007).
Choi, M.C. et al. Human microtubule-associated-protein tau regulates the number of protofilaments in microtubules: A synchrotron X-ray scattering study. Biophys. J. 97, 519–527 (2009).
Needleman, D. J. et al. Higher-order assembly of microtubules by counterions: From hexagonal bundles to living necklaces. Proc. Natl Acad. Sci. USA 101, 16099–16103 (2004).
Gelbart, W. M., Bruinsma, R. F., Pincus, P. A. & Parsegian, V. A. DNA-inspired electrostatics. Phys. Today 53, 38–44 (2000).
Koltover, I., Wagner, K. & Safinya, C. R. DNA condensation in two dimensions. Proc. Natl Acad. Sci. USA 97, 14046–14051 (2000).
Manning, G. S. Limiting laws and counterion condensation in polyelectrolyte solutions I. Colligative properties. J. Chem. Phys. 51, 924–933 (1969).
Savarin, P. et al. Central role for polyamines in microtubule assembly in cells. Biochem. J. 430, 151–159 (2010).
Jacobs, M., Bennett, P. M. & Dickens, M. J. Duplex microtubule is a new form of tubulin assembly induced by polycations. Nature 257, 707–709 (1975).
Erickson, H. P. & Voter, W. A. Polycation-induced assembly of purified tubulin. Proc. Natl Acad. Sci. USA 73, 2813–2817 (1976).
Kuznetsov, S. A., Gelfand, V. I., Rodionov, V. A., Rosenblat, V. A. & Gulyaeva, J. G. Polymerization of purified tubulin by synthetic polycations. FEBS Lett. 95, 343–346 (1978).
Erickson, H. P. in Cell Motility (eds Goldman, R., Pollard, T. & Rosenbaum, J.) (Cold Spring Harbor, 1976).
Howard, W. D. & Timasheff, S. N. GDP state of tubulin: Stabilization of double rings. Biochemistry 25, 8292–8300 (1986).
Nicholson, W. V., Lee, M., Downing, K. H. & Nogales, E. Cryo-electron microscopy of GDP-tubulin rings. Cell Biochem. Biophys. 31, 175–183 (1999).
Wang, H-W. & Nogales, E. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature 435, 911–915 (2005).
Raviv, U. et al. Cationic liposome-microtubule complexes: Pathways to the formation of two-state lipid-protein nanotubes with open or closed ends. Proc. Natl Acad. Sci. USA 102, 11167–11172 (2005).
Raviv, U. et al. Microtubule protofilament number is modulated in a stepwise fashion by the charge density of an enveloping layer. Biophys. J. 92, 278–287 (2007).
Cochran, W., Crick, F. H. C. & Vand, V. The structure of synthetic polypeptides. I. The transform of atoms on a helix. Acta Crystal. 5, 581–586 (1952).
Warren, B. E. X-ray diffraction in random layer lattice. Phys. Rev. 59, 693–698 (1941).
Glatter, O. & Kratky, O. (eds) in Small Angle X-ray Scattering 155–156 (Academic, 1982).
Levelut, A. Structure of a disk-like mesophase. J. Physique 40, 8184–8188 (1979).
Safinya, C. R., Liang, K. S., Varady, W. A., Clark, N. A. & Andersson, G. Synchrotron X-ray study of the orientational ordering D2-D1 structural phase transition of freely suspended discotic strands in triphenylene-hexa-dodecanoate. Phys. Rev. Lett. 53, 1172–1175 (1984).
Guinier, A. X-Ray Diffraction in Crystals, Imperfect Crystals, and Amorphous Bodies (Dover, 1963).
Goedert, M. & Spillantini, M. G. A century of Alzheimer’s disease. Science 314, 777–781 (2006).
Roberson, E. D. & Mucke, L. 100 Years and counting: Prospects for defeating Alzheimer’s disease. Science 314, 781–784 (2006).
Morris, M., Maeda, S., Vossel, K. & Mucke, L. The many faces of tau. Neuron 70, 410–426 (2011).
Kar, S., Fan, J., Smith, M. J., Goedert, M. & Amos, L. A. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 22, 70–77 (2003).
Makrides, V., Massie, M. R., Feinstein, S. C. & Lew, J. Evidence for two distinct binding sites for tau on microtubules. Proc. Natl Acad. Sci. USA 101, 6746–6751 (2004).
Miller, H. P. & Wilson, L. Preparation of microtubule protein and purified tubulin from bovine brain by cycles of assembly and disassembly and phosphocellulose chromatography. Methods Cell Biol. 95, 2–15 (2010).
Szekely, P., Ginsburg, A., Ben Nun, T. & Raviv, U. Solution X-ray scattering form factors of supramolecular self-assembled structures. Langmuir 26, 13110–13129 (2010).
Ben Nun, T., Ginsburg, A., Szekely, P. & Raviv, U. X+: A comprehensive, computationally accelerated, structural analysis tool of solution X-ray scattering from supramolecular self-assemblies. J. Appl. Crystallogr. 43, 1522–1531 (2010).
Acknowledgements
C.R.S., Y.L. and P.A.K. were supported by DOE-BES DE-FG02-06ER46314 (dynamic evolution of assemblies) and NSF DMR-1101900 (protein phase behaviour). L.W. and H.P.M. were supported by NIH R01-NS13560. D.J.N. and U.R. were supported by the US–Israel Binational Foundation (Grant 2009271), and U.R. acknowledges support from the Israel Science Foundation (Grant 1372/13). M.A.O-L. was supported by Mexico-based science foundations CONACyT, PIFI, PROMEP and UCMEXUS. C.S. was supported by National Research Foundation of Korea Grant NRF 2011-355-C00037. M.C.C. was supported by National Research Foundation of Korea Grants NRF 2011-0031931, 2011-0030923, 2012R1A1A1011023 and KAIST HRHRP N10110077. C.R.S. acknowledges discussions with KAIST faculty as part of his WCU (World Class University) Visiting Professor of Physics appointment supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology No. R33-2008-000-10163-0. We acknowledge use of UC-Santa Barbara’s TEM bioimaging and NSF-DMR-MRSEC facilities (NSF-DMR-1121053, a member of the NSF-funded Materials Research Facilities Network www.mrfn.org), and the Stanford Synchrotron Radiation Laboratory (SSRL), a DOE National Laboratory (where the SAXS work was performed).
Author information
Authors and Affiliations
Contributions
M.A.O-L., C.S. and M.C.C. performed electron microscopy, and M.A.O-L. and D.J.N. took X-ray data. H.P.M. purified tubulin. C.R.S., D.J.N., Y.L., U.R. and M.A.O-L. developed X-ray structure and form factors, and D.J.N. and A.G. carried out X-ray line-shape analysis. Y.L. and P.A.K. performed the statistical analysis of ring diameters, and Y.L. wrote the Supplementary Information. C.R.S., D.J.N. and M.A.O-L. wrote the paper. M.C.C., C.S., Y.L., U.R., H.P.M. and L.W. engaged in discussions and critical comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1633 kb)
Rights and permissions
About this article
Cite this article
Ojeda-Lopez, M., Needleman, D., Song, C. et al. Transformation of taxol-stabilized microtubules into inverted tubulin tubules triggered by a tubulin conformation switch. Nature Mater 13, 195–203 (2014). https://doi.org/10.1038/nmat3858
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3858
This article is cited by
-
Complexes of tubulin oligomers and tau form a viscoelastic intervening network cross-bridging microtubules into bundles
Nature Communications (2024)
-
An Overview on Taxol Production Technology and Its Applications as Anticancer Agent
Biotechnology and Bioprocess Engineering (2022)
-
Fibronectin type III domain-containing protein 5 promotes autophagy via the AMPK/mTOR signaling pathway in hepatocellular carcinoma cells, contributing to nab-paclitaxel chemoresistance
Medical Oncology (2022)
-
Progress in research on paclitaxel and tumor immunotherapy
Cellular & Molecular Biology Letters (2019)
-
Remote Controlled Autonomous Microgravity Lab Platforms for Drug Research in Space
Pharmaceutical Research (2019)