Abstract
Although postsynaptic glycine receptors (GlyRs) as αβ heteromers attract considerable research attention, little is known about the role of presynaptic GlyRs, likely α homomers, in diseases. Here, we demonstrate that dehydroxylcannabidiol (DH-CBD), a nonpsychoactive cannabinoid, can rescue GlyR functional deficiency and exaggerated acoustic and tactile startle responses in mice bearing point mutations in α1 GlyRs that are responsible for a hereditary startle-hyperekplexia disease. The GlyRs expressed as α1 homomers either in HEK-293 cells or at presynaptic terminals of the calyceal synapses in the auditory brainstem are more vulnerable than heteromers to hyperekplexia mutation–induced impairment. Homomeric mutants are more sensitive to DH-CBD than are heteromers, suggesting presynaptic GlyRs as a primary target. Consistent with this idea, DH-CBD selectively rescues impaired presynaptic GlyR activity and diminished glycine release in the brainstem and spinal cord of hyperekplexic mutant mice. Thus, presynaptic α1 GlyRs emerge as a potential therapeutic target for dominant hyperekplexia disease and other diseases with GlyR deficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Davidoff, R.A., Shank, R.P., Graham, L.T. Jr., Aprison, M.H. & Werman, R. Association of glycine with spinal interneurones. Nature 214, 680–681 (1967).
Betz, H. & Laube, B. Glycine receptors: recent insights into their structural organization and functional diversity. J. Neurochem. 97, 1600–1610 (2006).
Grudzinska, J. et al. The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 45, 727–739 (2005).
Weltzien, F., Puller, C., O'Sullivan, G.A., Paarmann, I. & Betz, H. Distribution of the glycine receptor β-subunit in the mouse CNS as revealed by a novel monoclonal antibody. J. Comp. Neurol. 520, 3962–3981 (2012).
Lynch, J.W. & Callister, R.J. Glycine receptors: a new therapeutic target in pain pathways. Curr. Opin. Investig. Drugs 7, 48–53 (2006).
Turecek, R. & Trussell, L.O. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature 411, 587–590 (2001).
Jeong, H.-J., Jang, I.-S., Moorhouse, A.J. & Akaike, N. Activation of presynaptic glycine receptors facilitates glycine release from presynaptic terminals synapsing onto rat spinal sacral dorsal commissural nucleus neurons. J. Physiol. (Lond.) 550, 373–383 (2003).
Ye, J.-H. et al. Presynaptic glycine receptors on GABAergic terminals facilitate discharge of dopaminergic neurons in ventral tegmental area. J. Neurosci. 24, 8961–8974 (2004).
Hruskova, B. et al. Differential distribution of glycine receptor subtypes at the rat calyx of held synapse. J. Neurosci. 32, 17012–17024 (2012).
Shiang, R. et al. Mutations in the α1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat. Genet. 5, 351–358 (1993).
Harvey, R.J., Topf, M., Harvey, K. & Rees, M.I. The genetics of hyperekplexia: more than startle!. Trends Genet. 24, 439–447 (2008).
Bakker, M.J., van Dijk, J.G., van den Maagdenberg, A.M. & Tijssen, M.A. Startle syndromes. Lancet Neurol. 5, 513–524 (2006).
Davies, J.S. et al. The glycinergic system in human startle disease: a genetic screening approach. Front. Mol. Neurosci. 3, 8 (2010).
Becker, L. et al. Disease-specific human glycine receptor α1 subunit causes hyperekplexia phenotype and impaired glycine- and GABA(A)-receptor transmission in transgenic mice. J. Neurosci. 22, 2505–2512 (2002).
Findlay, G.S. et al. Glycine receptor knock-in mice and hyperekplexia-like phenotypes: comparisons with the null mutant. J. Neurosci. 23, 8051–8059 (2003).
Blednov, Y.A., Benavidez, J.M., Homanics, G.E. & Harris, R.A. Behavioral characterization of knockin mice with mutations M287L and Q266I in the glycine receptor α1 subunit. J. Pharmacol. Exp. Ther. 340, 317–329 (2012).
Zhang, L. & Xiong, W. Nonpsychoactive cannabinoid action on 5-HT3 and glycine receptors. in Endocannabinoids: Actions at Non-CB1/CB2 Cannabinoid Receptors (eds. Abood, M.E., Sorensen, R.G. & Stella, N.) 199–218 (Springer, 2013).
Xiong, W. et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J. Exp. Med. 209, 1121–1134 (2012).
Xiong, W. et al. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat. Chem. Biol. 7, 296–303 (2011).
Kehne, J.H., Gallager, D.W. & Davis, M. Strychnine: brainstem and spinal mediation of excitatory effects on acoustic startle. Eur. J. Pharmacol. 76, 177–186 (1981).
Pribilla, I., Takagi, T., Langosch, D., Bormann, J. & Betz, H. The atypical M2 segment of the β subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 11, 4305–4311 (1992).
Yang, Z., Cromer, B.A., Harvey, R.J., Parker, M.W. & Lynch, J.W. A proposed structural basis for picrotoxinin and picrotin binding in the glycine receptor pore. J. Neurochem. 103, 580–589 (2007).
Deleuze, C. et al. Structural difference between heteromeric somatic and homomeric axonal glycine receptors in the hypothalamo-neurohypophysial system. Neuroscience 135, 475–483 (2005).
Schneggenburger, R. & Forsythe, I.D. The calyx of Held. Cell Tissue Res. 326, 311–337 (2006).
Turecek, R. & Trussell, L.O. Reciprocal developmental regulation of presynaptic ionotropic receptors. Proc. Natl. Acad. Sci. USA 99, 13884–13889 (2002).
O'Shea, S.M., Becker, L., Weiher, H., Betz, H. & Laube, B. Propofol restores the function of “hyperekplexic” mutant glycine receptors in Xenopus oocytes and mice. J. Neurosci. 24, 2322–2327 (2004).
Shan, Q., Han, L. & Lynch, J.W. Function of hyperekplexia-causing α1R271Q/L glycine receptors is restored by shifting the affected residue out of the allosteric signalling pathway. Br. J. Pharmacol. 165, 2113–2123 (2012).
Lape, R., Plested, A.J., Moroni, M., Colquhoun, D. & Sivilotti, L.G. The α1K276E startle disease mutation reveals multiple intermediate states in the gating of glycine receptors. J. Neurosci. 32, 1336–1352 (2012).
Harvey, R.J. et al. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887 (2004).
Zhou, H.Y. et al. N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J. Biol. Chem. 287, 33853–33864 (2012).
Andermann, F., Keene, D.L., Andermann, E. & Quesney, L.F. Startle disease or hyperekplexia: further delineation of the syndrome. Brain 103, 985–997 (1980).
Zhou, L., Chillag, K.L. & Nigro, M.A. Hyperekplexia: a treatable neurogenetic disease. Brain Dev. 24, 669–674 (2002).
Praveen, V., Patole, S.K. & Whitehall, J.S. Hyperekplexia in neonates. Postgrad. Med. J. 77, 570–572 (2001).
Rees, M.I. et al. Hyperekplexia associated with compound heterozygote mutations in the β-subunit of the human inhibitory glycine receptor (GLRB). Hum. Mol. Genet. 11, 853–860 (2002).
Chung, S.K. et al. GLRB is the third major gene of effect in hyperekplexia. Hum. Mol. Genet. 22, 927–940 (2013); erratum 22, 2552 (2013).
Rees, M.I. et al. Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat. Genet. 38, 801–806 (2006).
Izzo, A.A., Borrelli, F., Capasso, R., Di Marzo, V. & Mechoulam, R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 30, 515–527 (2009).
Ashton, H. Guidelines for the rational use of benzodiazepines. When and what to use. Drugs 48, 25–40 (1994).
Tijssen, M.A. et al. The effects of clonazepam and vigabatrin in hyperekplexia. J. Neurol. Sci. 149, 63–67 (1997).
Borghese, C.M. et al. Characterization of two mutations, M287L and Q266I, in the α1 glycine receptor subunit that modify sensitivity to alcohols. J. Pharmacol. Exp. Ther. 340, 304–316 (2012).
Kung, A.Y., Rick, C., O'Shea, S., Harrison, N.L. & McGehee, D.S. Expression of glycine receptors in rat sensory neurons vs. HEK293 cells yields different functional properties. Neurosci. Lett. 309, 202–206 (2001).
Sebe, J.Y., Eggers, E.D. & Berger, A.J. Differential effects of ethanol on GABAA and glycine receptor–mediated synaptic currents in brain stem motoneurons. J. Neurophysiol. 90, 870–875 (2003).
Chau, P., Hoifodt-Lido, H., Lof, E., Soderpalm, B. & Ericson, M. Glycine receptors in the nucleus accumbens involved in the ethanol intake-reducing effect of acamprosate. Alcohol. Clin. Exp. Res. 34, 39–45 (2010).
Li, J. et al. Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. J. Pharmacol. Exp. Ther. 341, 196–204 (2012).
Findlay, G.S. et al. Transgenic expression of a mutant glycine receptor decreases alcohol sensitivity of mice. J. Pharmacol. Exp. Ther. 300, 526–534 (2002).
Hu, X.-Q., Sun, H., Peoples, R.W., Hong, R. & Zhang, L. An interaction involving an arginine residue in the cytoplasmic domain of the 5-HT3A receptor contributes to receptor desensitization mechanism. J. Biol. Chem. 281, 21781–21788 (2006).
Pan, Y.Z. & Pan, H.L. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J. Neurophysiol. 91, 2413–2421 (2004).
Zhou, H.Y., Zhang, H.M., Chen, S.R. & Pan, H.L. Increased C-fiber nociceptive input potentiates inhibitory glycinergic transmission in the spinal dorsal horn. J. Pharmacol. Exp. Ther. 324, 1000–1010 (2008).
Helmchen, F., Borst, J.G. & Sakmann, B. Calcium dynamics associated with a single action potential in a CNS presynaptic terminal. Biophys. J. 72, 1458–1471 (1997).
Xiong, W., Wu, X., Lovinger, D.M. & Zhang, L. A common molecular basis for exogenous and endogenous cannabinoid potentiation of glycine receptors. J. Neurosci. 32, 5200–5208 (2012); erratum 32, 12979 (2012).
Acknowledgements
We thank A. Harris and Y. Blednov (University of Texas at Austin) for providing the α1 Q266I, α1 S267Q and α1 M287L mutant mice. We thank D.M. Lovinger for instrumental support and comments on the manuscript. This work was supported by funds from the intramural programs of the National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse and US National Institutes of Health grants to G.E.H. (AA10422) and from the National Institute of Neurological Disorders and Stroke to H.-L.P. (NS045602 and NS073935).
Author information
Authors and Affiliations
Contributions
W.X. and L.Z. conducted mutagenesis and animal behavioral tests. W.X. conducted patch-clamp recordings in HEK-293 cells. S.-R.C., Y.-L.Z., H.C., D.-P.L. and H.-L.P. conducted spinal slice recordings. L.H., W.X. and L.W. conducted brain stem calyceal recording. K.C. and K.C.R. synthesized DH-CBD. G.E.H. constructed genetically engineered mouse lines. J.P. provided the transgenic R271Q mouse line. W.X. and H.-L.P. participated in the study design and manuscript writing. L.Z. initiated, designed and supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The R271Q heterozygous mutant mice exhibit a rotarod performance similar to their wild type (WT) littermates.
The data representing the fall time of the R271Q mutant mice (solid squares, n=10) and WT littermates (open squares, n=10) in accelerating mode of rotorod test. All mice received 3 consecutive trials with 10 min intervals each day for three consecutive days. The experiments were carried out in a quiet and isolated room. These mice were handled gently.
Supplementary Figure 2 The efficacy of DH-CBD potentiation of R271Q mutant GlyRs.
Trace records of IGly before and after DH-CBD in HEK-293 cells expressing the α1R271Q mutant GlyRs. Concentration-response curve of DH-CBD potentiation of IGly. The EC50 value of DH-CBD potentiation is 5.7 ± 1.2 μM. Each data point represents mean±s.e. from at least 7 cells. The error bars invisible are smaller than the size of symbols.
Supplementary Figure 3 DH-CBD does not significantly alter strychnine inhibition of GlyRs.
(a). Gly concentration-response curves in the absence and presence of strychnine in HEK-293 cells expressing α1GlyRs. Strychnine at 0.1 μM produced in parallel a right shift of Gly concentration-response curve, respectively. The EC50 values are 56±8 μM for vehicle control (black squares, n=9) and 366±74 μM for strychnine treatment (blue squares, n=7). These values are significantly different (unpaired test, p=0.0023). ; DH-CBD did not significantly alter strychnine inhibition of the agonist binding affinity of α1GlyRs (red squares, n=7, Gly EC50: 368±84 μM. strychnine VS strychnine+DH-CBD , p=0.92) (b) Strychnine (n=6, 1 mg/kg, i.p.) produced exaggerated startle response to acoustic stimulation in C57/BL6 mice (p=0.006, two-way ANOVA). Preadministration of DH-CBD (n=6, 50 mg/kg, i.p.) did not reverse strychnine-induced exaggerated acoustic startle response (p=0.88, two-way ANOVA).
Supplementary Figure 4 Addition of the β subunit does not alter protein expression of R271Q and WT receptors at the cell surfaces
(a) Imaging of Western blot of total and surface proteins of GlyRs expressed in HEK-293 cells using the anti-α1 GlyR and anti-β-actin antiserums. (b) Quantitative analysis of the Western blot of GlyR proteins. Each bar represents mean±s.e. from separate 3 blots. (c) Full gel of surface GlyRs. (d) Full gel of total GlyRs. (e) Full gel of β-actin.
Supplementary Figure 5 DH-CBD does not restore diminished glycinergic transmission in spinal slices from the α1Q266I mutant mice.
(a) Trace records of Gly sIPSC in spinal slices from the WT and α1Q266I mutant mice with and without sustained perfusion of DH-CBD (20 μM) for 6 min. (b) The average frequency and amplitude of Gly sIPSC in spinal slices from the WT and α1Q266I mutant mice with and without sustained perfusion of DH-CBD (20 μM) for 6 min (n=10-12). WT VS Q266I, p=0.045, unpaired t-test; Q266I VS Q266I+DH-CBD, p=0.55, unpaired t-test. (c) The average amplitude of Gly sIPSC in spinal slices obtained from the WT and α1Q266I mutant mice with and without sustained perfusion of DH-CBD (20 μM) for 6 min (n=10-12). WT VS Q266I, p=0.048, unpaired t-test; Q266I VS Q266I+DH-CBD, p=0.63, unpaired t-test.
Supplementary Figure 6 The effect of PTX on the Gly sIPSC amplitdue in spinal slices obtained from the α1R271Q mutant mice.
The average amplitude of Gly sIPSC in spinal slices obtained from the mutant mice with and without sustained perfusion of DH-CBD (20 μM) for 6 min. Each bar represents the average from 8 cells.
Supplementary Figure 7 DH-CBD restores seizure-like behavior in homozygous M287L mice.
(a) Time course curves of seizure occurrence in homozygous M287L mice and wild type littermates during developmental stage. The seizure-like bebavior occurred after P12-14 in M287L mutant mice. Each data point represents the average lasting time (s) of seizure occurrence per min from 6-7 mice (Day 8-12, p>0.6, unpaired t-test; Day 14-32, p<0.05, unpaired t-test). (b) Time course curves of seizure occurrence after intraperitoneal injection of DH-CBD (50 mg/kg) in M287L mutant mice (P16-18). Each data point represents the average lasting time (s) of seizure occurrence per min from 5-7 mice. Time=10 min, WT VS M287L homo (homozygous), p=0.016, unpaired t-test; M287L homo+vehicle VS M287L homo+DH-CBD, p=0.0073, unpaired t-test.
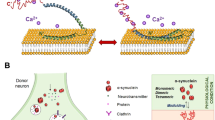
Supplementary Figure 8 Cannabinoid sensitive presynaptic GlyRs as a primary therapeutic target in the treatment of familial startle disease.
(a) Localization of tested hyperekplexia mutations in the α1 GlyR subunit. The mutations occurring in those GlyRs sensitive to cannabinoid are highlighted in blue, and the mutations occurring in those GlyRs insensitive to cannabinoid are highlighted in red. (b) Differential sensitivity of the mutant α1homomer and heteromer to cannabinoid-induced rescue of impaired GlyR Cl- channel activity. (c) Different states of presynaptic and postsynaptic mutant GlyRs in the presence or absence of cannabinoid in synapses containing hyperekplexic mutant α1 GlyRs.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 719 kb)
Supplementary Video 1
Exaggerated startle response to sound stimuli of the α1R271Q mutant mouse prior to DH-CBD administration. (MP4 4320 kb)
Supplementary Video 2
Exaggerated startle response to sound stimuli of the α1R271Q mutant mouse 5 min after administration of DH-CBD (30 mg/kg, i.p.). (MP4 1631 kb)
Supplementary Video 3
Delayed righting reflex of the α1R271Q mutant mouse prior to DH-CBD administration. (MP4 1798 kb)
Supplementary Video 4
Righting reflex of the α1R271Q mutant mouse 5 min after administration of DH-CBD (30 mg/kg, i.p.). (MP4 925 kb)
Rights and permissions
About this article
Cite this article
Xiong, W., Chen, SR., He, L. et al. Presynaptic glycine receptors as a potential therapeutic target for hyperekplexia disease. Nat Neurosci 17, 232–239 (2014). https://doi.org/10.1038/nn.3615
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3615
This article is cited by
-
Combined alcohol and cannabinoid exposure leads to synergistic toxicity by affecting cerebellar Purkinje cells
Nature Metabolism (2022)
-
Structural basis for cannabinoid-induced potentiation of alpha1-glycine receptors in lipid nanodiscs
Nature Communications (2022)
-
Glycine receptors and glycine transporters: targets for novel analgesics?
Cellular and Molecular Life Sciences (2018)
-
Expression of glycine receptor alpha 3 in the rat trigeminal neurons and central boutons in the brainstem
Brain Structure and Function (2016)
-
Molecular Targets of Cannabidiol in Neurological Disorders
Neurotherapeutics (2015)