Key Points
-
Aurora/Ipl1-related kinases are evolutionally conserved serine/threonine kinases that regulate mitotic progression in various organisms. Humans have three classes of Aurora kinases (A, B and C). Aurora-A and -B are ubiquitously expressed and regulate cell-cycle events from G2 to M phase.
-
Aurora-A is localized at centrosome during interphase, translocated to mitotic spindles in early mitotic phase and degraded after metaphase–anaphase transition. Activation of Aurora-A is required for mitotic entry, centrosome maturation, centrosome separation and chromosome alignment, and inactivation is also necessary for exit from mitosis.
-
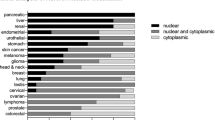
Human Aurora-A is frequently amplified in various cancers. The levels of Aurora-A mRNA and protein are increased in those tumours and the overexpression of Aurora-A efficiently transforms immortalized rodent fibroblasts, indicating that Aurora-A is an oncoprotein.
-
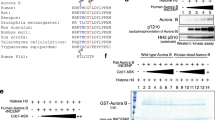
Aurora-A kinase is activated by interaction with Ajuba and TPX2 during late G2 and mitotic phases, respectively.
-
Overexpression of Aurora-A induces abnormalities in G2 checkpoint and spindle checkpoint and cytokinesis failure. Those abnormalities lead to chromosome instability but are not sufficient for tumorigenesis in animal models. Additional changes such as p53 inactivation and expression of pro-survival proteins might be required for Aurora-A-mediated tumorigenesis.
-
Aurora-kinase inhibition effectively blocks cell growth and induces apoptosis in cancer cells. It might provide a new approach for the treatment of many human malignancies.
Abstract
The three human homologues of Aurora kinases (A, B and C) are essential for proper execution of various mitotic events and are important for maintaining genomic integrity. Aurora-A is mainly localized at spindle poles and the mitotic spindle during mitosis, where it regulates the functions of centrosomes, spindles and kinetochores required for proper mitotic progression. Recent studies have revealed that Aurora-A is frequently overexpressed in various cancer cells, indicating its involvement in tumorigenesis. What are the normal physiological roles of Aurora-A, how are these regulated and how might the enzyme function during tumorigenesis?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nigg, E. A. Mitotic kinases as regulators of cell division and its checkpoints. Nature Rev. Mol. Cell Biol. 2, 21–32 (2001).
Li, X. et al. Direct association with inner centromere protein (INCENP) activates the novel chromosomal passenger protein, Aurora-C. J. Biol. Chem. 279, 47201–47211 (2004).
Hu, H. M., Chuang, C. K., Lee, M. J., Tseng, T. C. & Tang, T. K. Genomic organization, expression, and chromosome localization of a third aurora-related kinase gene, Aie1. DNA Cell Biol. 19, 679–688 (2000).
Bischoff, J. R. et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17, 3052–3065 (1998). Demonstrates that Aurora A is overexpressed in more than 50% of primary colorectal cancers, and that overexpression of Aurora A transforms rodent fibroblasts.
Zhou, H. et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nature Genet. 20, 189–193 (1998).
Tanner, M. M. et al. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin. Cancer Res. 6, 1833–1839 (2000).
Watanabe, T. et al. Differentially regulated genes as putative targets of amplifications at 20q in ovarian cancers. Jpn J. Cancer Res. 93, 1114–1122 (2002).
Rojanala, S. et al. The mitotic serine threonine kinase, Aurora-2, is a potential target for drug development in human pancreatic cancer. Mol. Cancer Ther. 3, 451–457 (2004).
Tanaka, T. et al. Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res. 59, 2041–2044 (1999).
Takahashi, T. et al. Centrosomal kinases, HsAIRK1 and HsAIRK3, are overexpressed in primary colorectal cancers. Jpn J. Cancer Res. 91, 1007–1014 (2000).
Gritsko, T. M. et al. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin. Cancer Res. 9, 1420–1426 (2003).
Li, D. et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin. Cancer Res. 9, 991–997 (2003).
Katayama, H. et al. Mitotic kinase expression and colorectal cancer progression. J. Natl Cancer Inst. 91, 1160–1162 (1999).
Tatsuka, M. et al. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 58, 4811–4816 (1998).
Ota, T. et al. Increased mitotic phosphorylation of histone H3 attributable to AIM-1/Aurora-B overexpression contributes to chromosome number instability. Cancer Res. 62, 5168–5177 (2002).
Kimura, M., Matsuda, Y., Yoshioka, T. & Okano, Y. Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3. J. Biol. Chem. 274, 7334–7340 (1999).
Hannak, E., Kirkham, M., Hyman, A. A. & Oegema, K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155, 1109–1116 (2001).
Giet, R. et al. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 156, 437–451 (2002).
Berdnik, D. & Knoblich, J. A. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr. Biol. 12, 640–647 (2002).
Hirota, T. et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell 114, 585–598 (2003).
Bellanger, J. M. & Gonczy, P. TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr. Biol. 13, 1488–1498 (2003).
Conte, N. et al. TACC1–chTOG–Aurora A protein complex in breast cancer. Oncogene 22, 8102–8116 (2003).
Marumoto, T. et al. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J. Biol. Chem. 278, 51786–51795 (2003). References 20 and 23 describe the subcellular localization of Aurora-A during the cell cycle and show that Aurora-A activity is required for execution of various mitotic events, such as mitotic entry, centrosome maturation, centrosome separation, chromosome alignment and cytokinesis.
Giet, R., Uzbekov, R., Cubizolles, F., Le Guellec, K. & Prigent, C. The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J. Biol. Chem. 274, 15005–15013 (1999).
Dutertre, S. et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J. Cell Sci. 117, 2523–2531 (2004).
Marie, H. et al. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with α-catenin. J. Biol. Chem. 278, 1220–1228 (2003).
Roghi, C. et al. The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 111, 557–572 (1998).
Tsai, M. Y. et al. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nature Cell Biol. 5, 242–248 (2003).
Kalab, P., Pu, R. T. & Dasso, M. The ran GTPase regulates mitotic spindle assembly. Curr. Biol. 9, 481–484 (1999).
Carazo-Salas, R. E. et al. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400, 178–181 (1999).
Wittmann, T., Boleti, H., Antony, C., Karsenti, E. & Vernos, I. Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein. J. Cell Biol. 143, 673–685 (1998).
Karsenti, E. & Vernos, I. The mitotic spindle: a self-made machine. Science 294, 543–547 (2001).
Wittmann, T., Wilm, M., Karsenti, E. & Vernos, I. TPX2, A novel xenopus MAP involved in spindle pole organization. J. Cell Biol. 149, 1405–1418 (2000).
Garrett, S., Auer, K., Compton, D. A. & Kapoor, T. M. hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr. Biol. 12, 2055–2059 (2002).
Kufer, T. A. et al. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 158, 617–623 (2002).
Eyers, P. A., Erikson, E., Chen, L. G. & Maller, J. L. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 13, 691–697 (2003). References 35 and 36 show that TPX2 interacts with and is phosphorylated by Aurora-A, and acts as an activator of Aurora-A.
Giet, R. & Prigent, C. The Xenopus laevis aurora/Ip11p-related kinase pEg2 participates in the stability of the bipolar mitotic spindle. Exp. Cell Res. 258, 145–151 (2000).
Ditchfield, C. et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267–280 (2003).
Hauf, S. et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281–294 (2003).
Andrews, P. D., Knatko, E., Moore, W. J. & Swedlow, J. R. Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 15, 672–683 (2003).
Kunitoku, N. et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev. Cell 5, 853–864 (2003).
Cheung, P., Allis, C. D. & Sassone-Corsi, P. Signaling to chromatin through histone modifications. Cell 103, 263–271 (2000).
Zeitlin, S. G., Shelby, R. D. & Sullivan, K. F. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J. Cell Biol. 155, 1147–1157 (2001).
Meraldi, P., Honda, R. & Nigg, E. A. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 21, 483–492 (2002).
Anand, S., Penrhyn-Lowe, S. & Venkitaraman, A. R. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 3, 51–62 (2003). Reports that overexpression of Aurora-A disrupts the spindle checkpoint, resulting in resistance to apoptosis induced by paclitaxel in a human cancer cell line.
Zhang, D. et al. Cre–loxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene 23, 8720–8730 (2004).
Honda, K. et al. Degradation of human Aurora2 protein kinase by the anaphase-promoting complex-ubiquitin-proteasome pathway. Oncogene 19, 2812–2819 (2000).
Castro, A. et al. The D-Box-activating domain (DAD) is a new proteolysis signal that stimulates the silent D-Box sequence of Aurora-A. EMBO Rep. 3, 1209–1214 (2002).
Jeng, Y. M., Peng, S. Y., Lin, C. Y. & Hsu, H. C. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin. Cancer Res. 10, 2065–2071 (2004).
Sakakura, C. et al. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br. J. Cancer 84, 824–831 (2001).
Goepfert, T. M. et al. Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res. 62, 4115–4122 (2002).
Miyoshi, Y., Iwao, K., Egawa, C. & Noguchi, S. Association of centrosomal kinase STK15/BTAK mRNA expression with chromosomal instability in human breast cancers. Int. J. Cancer 92, 370–373 (2001).
Marumoto, T. et al. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells 7, 1173–1182 (2002).
Jiang, Y., Zhang, Y., Lees, E. & Seghezzi, W. AuroraA overexpression overrides the mitotic spindle checkpoint triggered by nocodazole, a microtubule destabilizer. Oncogene 22, 8293–8301 (2003).
Shackney, S. E. et al. Model for the genetic evolution of human solid tumors. Cancer Res. 49, 3344–3354 (1989).
Minn, A. J., Boise, L. H. & Thompson, C. B. Expression of Bcl-XL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 10, 2621–2631 (1996).
Lanni, J. S. & Jacks, T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol. Cell. Biol. 18, 1055–1064 (1998).
Rieder, C. L. & Maiato, H. Stuck in division or passing through; what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7, 637–651 (2004).
Andreassen, P. R., Lohez, O. D., Lacroix, F. B. & Margolis, R. L. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol. Biol. Cell 12, 1315–1328 (2001).
Margolis, R. L., Lohez, O. D. & Andreassen, P. R. G1 tetraploidy checkpoint and the suppression of tumorigenesis. J. Cell Biochem. 88, 673–683 (2003).
Di Leonardo, A. et al. DNA rereplication in the presence of mitotic spindle inhibitors in human and mouse fibroblasts lacking either p53 or pRb function. Cancer Res. 57, 1013–1019 (1997).
Khan, S. H. & Wahl, G. M. p53 and pRb prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res. 58, 396–401 (1998).
Casenghi, M. et al. p53-independent apoptosis and p53-dependent block of DNA rereplication following mitotic spindle inhibition in human cells. Exp. Cell Res. 250, 339–350 (1999).
Stewart, Z. A., Leach, S. D. & Pietenpol, J. A. p21Waf1/Cip1 inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol. Cell. Biol. 19, 205–215 (1999).
Vogel, C., Kienitz, A., Hofmann, I., Muller, R. & Bastians, H. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene 23, 6845–6853 (2004).
Chen, S. S., Chang, P. C., Cheng, Y. W., Tang, F. M. & Lin, Y. S. Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. EMBO J. 21, 4491–4499 (2002).
Katayama, H. et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nature Genet. 36, 55–62 (2004).
Liu, Q. et al. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine-215. J. Biol. Chem. 6 Oct 2004 (doi:10.1074/jbc.M406802200).
Ewart-Toland, A. et al. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nature Genet. 34, 403–412 (2003).
DiCioccio, R. A. et al. STK15 polymorphisms and association with risk of invasive ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 13, 1589–1594 (2004).
Egan, K. M. et al. STK15 polymorphism and breast cancer risk in a population-based study. Carcinogenesis 25, 2149–2153 (2004).
Harrington, E. A. et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nature Med. 10, 262–267 (2004). Describes a small-molecule inhibitor of Aurora kinases, VX-680, that blocks cell-cycle progression and induces apoptosis in various human tumour cell types, both in vitro and in vivo.
Dutertre, S. & Prigent, C. Aurora-A overexpression leads to override of the microtubule-kinetochore attachment checkpoint. Mol. Interv. 3, 127–130 (2003).
Vogelstein, B. & Kinzler, K. W. Cancer genes and the pathways they control. Nature Med. 10, 789–799 (2004).
Yang, H. et al. Aurora-A kinase regulates telomerase activity through c-Myc in human ovarian and breast epithelial cells. Cancer Res. 64, 463–467 (2004).
Kimura, M. et al. Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1. J. Biol. Chem. 272, 13766–13771 (1997).
Bischoff, J. R. & Plowman, G. D. The Aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol. 9, 454–459 (1999).
Glover, D. M., Leibowitz, M. H., McLean, D. A. & Parry, H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 81, 95–105 (1995).
Kim, J. H., Kang, J. S. & Chan, C. S. Sli15 associates with the ipl1 protein kinase to promote proper chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol. 145, 1381–1394 (1999).
Adams, R. R. et al. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 10, 1075–1078 (2000).
Kaitna, S., Mendoza, M., Jantsch-Plunger, V. & Glotzer, M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 10, 1172–1181 (2000).
Terada, Y. et al. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 17, 667–676 (1998).
Giet, R. & Glover, D. M. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152, 669–682 (2001).
Severson, A. F., Hamill, D. R., Carter, J. C., Schumacher, J. & Bowerman, B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 10, 1162–1171 (2000).
Murata-Hori, M. et al. Myosin II regulatory light chain as a novel substrate for AIM-1, an aurora/Ipl1p-related kinase from rat. J. Biochem. (Tokyo) 128, 903–907 (2000).
Goto, H. et al. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J. Biol. Chem. 278, 8526–8530 (2003).
Kawajiri, A. et al. Functional significance of the specific sites phosphorylated in desmin at cleavage furrow: Aurora-B may phosphorylate and regulate type III intermediate filaments during cytokinesis coordinatedly with Rho-kinase. Mol. Biol. Cell 14, 1489–1500 (2003).
Minoshima, Y. et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev. Cell 4, 549–560 (2003).
Kawasaki, A. et al. Downregulation of an AIM-1 kinase couples with megakaryocytic polyploidization of human hematopoietic cells. J. Cell Biol. 152, 275–287 (2001).
Geddis, A. E. & Kaushansky, K. Megakaryocytes express functional Aurora-B kinase in endomitosis. Blood 104, 1017–1024 (2004).
Mendez, R. et al. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404, 302–307 (2000).
Castro, A., Mandart, E., Lorca, T. & Galas, S. Involvement of Aurora A kinase during meiosis I-II transition in Xenopus oocytes. J. Biol. Chem. 278, 2236–2241 (2003).
Katayama, H., Zhou, H., Li, Q., Tatsuka, M. & Sen, S. Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle. J. Biol. Chem. 276, 46219–46224 (2001).
Bishop, J. D. & Schumacher, J. M. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J. Biol. Chem. 277, 27577–27580 (2002).
Rogers, E., Bishop, J. D., Waddle, J. A., Schumacher, J. M. & Lin, R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J. Cell Biol. 157, 219–129 (2002).
Lan, W. et al. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14, 273–286 (2004).
Gigoux, V., L'Hoste, S., Raynaud, F., Camonis, J. & Garbay, C. Identification of Aurora kinases as RasGAP Src homology 3 domain-binding proteins. J. Biol. Chem. 277, 23742–23746 (2002).
Du, J. & Hannon, G. J. The centrosomal kinase Aurora-A/STK15 interacts with a putative tumor suppressor NM23-H1. Nucleic Acids Res. 30, 5465–5475 (2002).
Littlepage, L. E. & Ruderman, J. V. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 16, 2274–2285 (2002).
Acknowledgements
We thank T. Hirota, T. Sasayama, S. Iida, S. Kuninaka, T. Hara, N. Kunitoku, N. Araki and T. Mimori for helpful discussion. This work was supported by a grant for Cancer Research from the Ministry of Education, Science and Culture of Japan (H.S.) and 'Research for the Future' programme of the Japan Society for the Promotion of Science (H.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
National Cancer Institute
FURTHER INFORMATION
Glossary
- LIM PROTEINS
-
LIM domains are modular protein-interaction motifs present in proteins with diverse functions. Ajuba has three LIM domains at the carboxyl terminus, and LIM-2 and LIM-3 domains are required for interaction with Aurora-A.
- KINETOCHORE
-
Kinetochores are required for normal chromosome alignment. They provide a site for the attachment of spindle microtubules that allows generation of the force required for chromosome movement.
- AMPHITELIC ATTACHMENT STATE
-
During mitosis, to achieve the proper segregation of replicated chromosomes, the two sister kinetochores on each chromosome need to attach to microtubules of opposite polarity.
- CENP-A
-
CENP-A is a conserved variant of histone H3 that substitutes for this protein in the nucleosome core of centromeric chromatin. CENP-A is required for assembly of other kinetochore proteins and is required for chromosome alignment at the metaphase plate.
Rights and permissions
About this article
Cite this article
Marumoto, T., Zhang, D. & Saya, H. Aurora-A — A guardian of poles. Nat Rev Cancer 5, 42–50 (2005). https://doi.org/10.1038/nrc1526
Issue Date:
DOI: https://doi.org/10.1038/nrc1526
This article is cited by
-
Aurora kinase A-mediated phosphorylation triggers structural alteration of Rab1A to enhance ER complexity during mitosis
Nature Structural & Molecular Biology (2024)
-
Novel target and treatment agents for natural killer/T-cell lymphoma
Journal of Hematology & Oncology (2023)
-
Epigenetic effects of herbal medicine
Clinical Epigenetics (2023)
-
Targeting Aurora-A inhibits tumor progression and sensitizes thyroid carcinoma to Sorafenib by decreasing PFKFB3-mediated glycolysis
Cell Death & Disease (2023)
-
LncRNA TIALD contributes to hepatocellular carcinoma metastasis via inducing AURKA lysosomal degradation
Cell Death Discovery (2023)