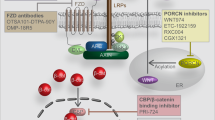
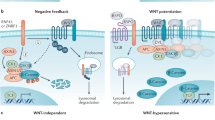
Key Points
-
WNTs are secreted glycoproteins that regulate multiple signalling pathways through both β-catenin (CTNNB1)-dependent and CTNNB1–independent mechanisms.
-
The activation of WNT signalling pathways can be both positively and negatively correlated with patient outcomes in different types of cancer.
-
WNT–CTNNB1 signalling can either promote or inhibit tumour initiation, growth, metastases and drug resistance in a cancer-stage-specific and a cancer-type-specific manner.
-
CTNNB1-independent WNT signalling pathways also contribute to tumorigenesis and cancer progression in a context-dependent manner.
-
Aberrations in WNT signalling pathways and alterations in other oncogene and tumour suppressor pathways cooperate to drive cancer initiation and progression.
-
Multiple strategies for targeting WNT signalling — ranging from small molecules to blocking antibodies, and peptide agonists and antagonists — are now in development, thus paving the way for initial clinical trials using WNT modulators in cancer patients.
Abstract
Since the initial discovery of the oncogenic activity of WNT1 in mouse mammary glands, our appreciation for the complex roles for WNT signalling pathways in cancer has increased dramatically. WNTs and their downstream effectors regulate various processes that are important for cancer progression, including tumour initiation, tumour growth, cell senescence, cell death, differentiation and metastasis. Although WNT signalling pathways have been difficult to target, improved drug-discovery platforms and new technologies have facilitated the discovery of agents that can alter WNT signalling in preclinical models, thus setting the stage for clinical trials in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Papkoff, J., Brown, A. M. & Varmus, H. E. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol. Cell. Biol. 7, 3978–3984 (1987).
Nusse, R. & Varmus, H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31, 99–109 (1982). This seminal study identified a role for the Wnt1 locus (also known as int1 ) in the regulation of mammary tumorigenesis in mice.
Nusse, R., Van Ooyen, A., Cox, D., Fung, Y. K. & Varmus, H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature 307, 131–136 (1984).
Tsukamoto, A. S., Grosschedl, R., Guzman, R. C., Parslow, T. & Varmus, H. E. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55, 619–625 (1988).
Papkoff, J., Rubinfeld, B., Schryver, B. & Polakis, P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol. Cell. Biol. 16, 2128–2134 (1996).
Korinek, V. et al. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 (1997).
Morin, P. J. et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275, 1787–1790 (1997).
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Segditsas, S. & Tomlinson, I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25, 7531–7537 (2006).
Angers, S. & Moon, R. T. Proximal events in Wnt signal transduction. Nature Rev. Mol. Cell. Biol. 10, 468–477 (2009).
Kohn, A. D. & Moon, R. T. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium 38, 439–446 (2005).
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Bass, A. J. et al. Genomic sequencing of colorectal adenocarcinomas identifies a recurrent VTI1A–TCF7L2 fusion. Nature Genet. 43, 964–968 (2011).
Laurent-Puig, P. & Zucman-Rossi, J. Genetics of hepatocellular tumors. Oncogene 25, 3778–3786 (2006).
Breuhahn, K., Longerich, T. & Schirmacher, P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene 25, 3787–3800 (2006).
Zurawel, R. H., Chiappa, S. A., Allen, C. & Raffel, C. Sporadic medulloblastomas contain oncogenic β-catenin mutations. Cancer Res. 58, 896–899 (1998).
Palacios, J. & Gamallo, C. Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 58, 1344–1347 (1998).
Salahshor, S. & Woodgett, J. R. The links between axin and carcinogenesis. J. Clin. Pathol. 58, 225–236 (2005).
Satoh, S. et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nature Genet. 24, 245–250 (2000).
Mani, A. et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315, 1278–1282 (2007).
Robitaille, J. et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nature Genet. 32, 326–330 (2002).
De Ferrari, G. V. et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer's disease. Proc. Natl Acad. Sci. USA 104, 9434–9439 (2007).
Chim, C. S., Pang, R., Fung, T. K., Choi, C. L. & Liang, R. Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia 21, 2527–2536 (2007).
Klarmann, G. J., Decker, A. & Farrar, W. L. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics 3, 59–63 (2008).
Aguilera, O. et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 25, 4116–4121 (2006).
Kansara, M. et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J. Clin. Invest. 119, 837–851 (2009).
Baldus, S. E. et al. MUC1 and nuclear β-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin. Cancer Res. 10, 2790–2796 (2004).
Lin, S. Y. et al. β-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc. Natl Acad. Sci. USA 97, 4262–4266 (2000).
Cheah, P. Y., Choo, P. H., Yao, J., Eu, K. W. & Seow-Choen, F. A survival-stratification model of human colorectal carcinomas with β-catenin and p27kip1. Cancer 95, 2479–2486 (2002).
Brabletz, T. et al. Nuclear overexpression of the oncoprotein β-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol. Res. Pract. 194, 701–704 (1998).
Cheng, H., Liang, H., Qin, Y. & Liu, Y. Nuclear β-catenin overexpression in metastatic sentinel lymph node is associated with synchronous liver metastasis in colorectal cancer. Diagn. Pathol. 6, 109 (2011).
Chung, G. G. et al. Tissue microarray analysis of β-catenin in colorectal cancer shows nuclear phospho-β-catenin is associated with a better prognosis. Clin. Cancer Res. 7, 4013–4020 (2001).
Bukholm, I. K., Nesland, J. M., Kåresen, R., Jacobsen, U. & Børresen-Dale, A. L. E-cadherin and α-, β-, and γ-catenin protein expression in relation to metastasis in human breast carcinoma. J. Pathol. 185, 262–266 (1998).
Khramtsov, A. I. et al. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 176, 2911–2920 (2010).
López-Knowles, E. et al. Cytoplasmic localization of β-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol. Biomarkers Prev. 19, 301–309 (2010).
Fattet, S. et al. β-catenin status in paediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J. Pathol. 218, 86–94 (2009).
Ellison, D. W. et al. β-catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J. Clin. Oncol. 23, 7951–7957 (2005). References 36 and 37 show that active WNT–CTNNB1 signalling is associated with lower-risk medulloblastoma tumours compared with mutations in the SHH pathway.
Horvath, L. G. et al. Lower levels of nuclear β-catenin predict for a poorer prognosis in localized prostate cancer. Int. J. Cancer 113, 415–422 (2005).
Gamallo, C. et al. β-catenin expression pattern in stage I and II ovarian carcinomas: relationship with β-catenin gene mutations, clinicopathological features, and clinical outcome. Am. J. Pathol. 155, 527–536 (1999).
Chien, A. J. et al. Activated Wnt/β-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc. Natl Acad. Sci. USA 106, 1193–1198 (2009).
Bittner, M. et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406, 536–540 (2000).
Weeraratna, A. T. et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1, 279–288 (2002). This study identifies an important role for WNT5A in regulating melanoma invasiveness in a PKC-dependent but CTNNB1-independent manner.
Da Forno, P. D. et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin. Cancer Res. 14, 5825–5832 (2008). This paper suggests that WNT5A potentially drives melanoma metastasis and that WNT5A expression is increased in aggressive melanomas, resulting in patient deaths and metastasis.
Kurayoshi, M. et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 66, 10439–10448 (2006).
Lejeune, S., Huguet, E. L., Hamby, A., Poulsom, R. & Harris, A. L. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin. Cancer Res. 1, 215–222 (1995).
Dejmek, J., Dejmek, A., Säfholm, A., Sjölander, A. & Andersson, T. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 65, 9142–9146 (2005).
Wong, G. T., Gavin, B. J. & McMahon, A. P. Differential transformation of mammary epithelial cells by Wnt genes. Mol. Cell. Biol. 14, 6278–6286 (1994).
Shimizu, H. et al. Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ. 8, 1349–1358 (1997).
Naylor, S. et al. Retroviral expression of Wnt-1 and Wnt-7b produces different effects in mouse mammary epithelium. J. Cell. Sci. 113, 2129–2138 (2000).
Qiang, Y.-W., Endo, Y., Rubin, J. S. & Rudikoff, S. Wnt signaling in B-cell neoplasia. Oncogene 22, 1536–1545 (2003).
Derksen, P. W. B. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc. Natl Acad. Sci. 101, 6122–6127 (2004).
Verras, M., Brown, J., Li, X., Nusse, R. & Sun, Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 64, 8860–8866 (2004).
Li, X. et al. Prostate tumor progression is mediated by a paracrine TGF-β/Wnt3a signaling axis. Oncogene 27, 7118–7130 (2008).
Pearson, H. B., Phesse, T. J. & Clarke, A. R. K-ras and Wnt Signaling synergize to accelerate prostate tumorigenesis in the mouse. Cancer Res. 69, 94–101 (2009).
Yu, X., Wang, Y., DeGraff, D. J., Wills, M. L. & Matusik, R. J. Wnt/β-Catenin activation promotes prostate tumor progression in a mouse model. Oncogene 30, 1868–1879 (2010).
Biechele, T. L. et al. Wnt/β-catenin signaling and AXIN1 regulate apoptosis triggered by inhibition of the mutant kinase BRAFV600E in human melanoma. Sci. Signal. 5, ra3 (2012).
Yoshioka, S. et al. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/β-catenin pathway. Mol. Cancer Res. 10, 469–482 (2012).
Ochoa-Hernández, A. B. et al. Peripheral T-lymphocytes express WNT7A and its restoration in leukemia-derived lymphoblasts inhibits cell proliferation. BMC Cancer 12, 60 (2012).
Carmon, K. S. & Loose, D. S. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol. Cancer Res. 6, 1017–1028 (2008).
Guturi, K. K. N. et al. Mechanism of β-catenin-mediated transcriptional regulation of epidermal growth factor receptor expression in glycogen synthase kinase 3β-inactivated prostate cancer cells. J. Biol. Chem. 287, 18287–18296 (2012).
Bitler, B. G. et al. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res. 71, 6184–6194 (2011).
Kremenevskaja, N. et al. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 24, 2144–2154 (2005).
Liang, H. et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 4, 349–360 (2003).
Schwartz, A. L. et al. Phenylmethimazole decreases toll-like receptor 3 and noncanonical Wnt5a expression in pancreatic cancer and melanoma together with tumor cell growth and migration. Clin. Cancer Res. 15, 4114–4122 (2009).
Fukuda, T. et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc. Natl Acad. Sci. 105, 3047–3052 (2008).
Wu, A. et al. Persistence of CD133+ cells in human and mouse glioma cell lines: detailed characterization of GL261 glioma cells with cancer stem cell-like properties. Stem Cells Dev. 17, 173–184 (2008).
King, T. D., Zhang, W., Suto, M. J. & Li, Y. Frizzled7 as an emerging target for cancer therapy. Cell. Signal. 24, 846–851 (2012).
Yang, L. et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 30, 4437–4446 (2011).
Wei, W., Chua, M.-S., Grepper, S. & So, S. K. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol. Cancer 10, 16 (2011).
Liu, J. et al. Enhancement of canonical Wnt/β-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PLoS ONE 6, e27496 (2011).
Yamaguchi, T. et al. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell 21, 348–361 (2012). Results from this study highlight a particularly robust role for ROR1 in promoting lung tumorigenesis through crosstalk with receptor-tyrosine-kinase-dependent signalling pathways.
Zhang, S. et al. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS ONE 7, e31127 (2012).
Gentile, A., Lazzari, L., Benvenuti, S., Trusolino, L. & Comoglio, P. M. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer Res. 71, 3132–3141 (2011).
Wright, T. M. et al. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene 28, 2513–2523 (2009).
Edris, B. et al. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J. Pathol. 227, 223–233 (2012).
Schlange, T., Matsuda, Y., Lienhard, S., Huber, A. & Hynes, N. E. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res. 9, R63 (2007).
Matsuda, Y., Schlange, T., Oakeley, E. J., Boulay, A. & Hynes, N. E. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 11, R32 (2009).
DiMeo, T. A. et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 69, 5364–5373 (2009).
Horvath, L. G. et al. Membranous expression of secreted frizzled-related protein 4 predicts for good prognosis in localized prostate cancer and inhibits PC3 cellular proliferation in vitro. Clin. Cancer Res. 10, 615–625 (2004).
Zi, X. et al. Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res. 65, 9762–9770 (2005).
Joesting, M. S. et al. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 65, 10423–10430 (2005).
Yee, D. S. et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol. Cancer 9, 162 (2010).
Lambiv, W. L. et al. The Wnt inhibitory factor 1 (WIF1) is targeted in glioblastoma and has a tumor suppressing function potentially by induction of senescence. Neuro Oncol. 13, 736–747 (2011).
Ramachandran, I. et al. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene 31, 2725–2737 (2011).
Rubin, E. M. et al. Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcoma. Mol. Cancer Ther. 9, 731–741 (2010).
Nguyen, L. V., Vanner, R., Dirks, P. & Eaves, C. J. Cancer stem cells: an evolving concept. Nature Rev. Cancer 12, 133–143 (2012).
Yeung, J. et al.β-catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 18, 606–618 (2010).
Jamieson, C. H. M. et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351, 657–667 (2004). This paper shows that nuclear CTNNB1 and CTNNB1-dependent transcription is upregulated in drug-resistant CML and in CML with blast crisis, suggesting that CTNNB1 may be upregulated in leukaemia progenitor-like cells in patients.
Zhao, C. et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell 12, 528–541 (2007).
Wang, Y. et al. The Wnt/β-catenin pathway is required for the development of leukemia stem cells in AML. Science 327, 1650–1653 (2010).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009). This study finds that activating WNT signalling by deleting Apc in a subset of colon progenitor cells induces the rapid development of adenocarcinomas, whereas deleting Apc in other cells does not induce tumours. This indicates that activation of CTNNB1 signalling in progenitor cells drives tumorigenesis.
Van de Wetering, M. et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002).
Deka, J. et al. Bcl9/Bcl9l are critical for Wnt-mediated regulation of stem cell traits in colon epithelium and adenocarcinomas. Cancer Res. 70, 6619–6628 (2010).
Steg, A. D. et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin. Cancer Res. 18, 869–881 (2011).
Li, Y. et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc. Natl Acad. Sci. USA 100, 15853–15858 (2003).
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006).
Hallett, R. M. et al. Small molecule antagonists of the Wnt/β-catenin signaling pathway target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. PLoS ONE 7, e33976 (2012).
Zeng, Y. A. & Nusse, R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6, 568–577 (2010).
Scheel, C. et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145, 926–940 (2011).
Ye, X. et al. Downregulation of Wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Mol. Cell 27, 183–196 (2007).
Zhang, H. et al. Interference of Frizzled 1 (FZD1) reverses multidrug resistance in breast cancer cells through the Wnt/β-catenin pathway. Cancer Lett. 323, 106–113 (2012).
Noda, T. et al. Activation of Wnt/β-catenin signalling pathway induces chemoresistance to interferon-α/5-fluorouracil combination therapy for hepatocellular carcinoma. Br. J. Cancer 100, 1647–1658 (2009).
Flahaut, M. et al. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/β-catenin pathway. Oncogene 28, 2245–2256 (2009).
Bordonaro, M., Tewari, S., Cicco, C. E., Atamna, W. & Lazarova, D. L. A switch from canonical to noncanonical Wnt signaling mediates drug resistance in colon cancer cells. PLoS ONE 6, e27308 (2011).
Zhou, Z., Wang, J., Han, X., Zhou, J. & Linder, S. Up-regulation of human secreted frizzled homolog in apoptosis and its down-regulation in breast tumors. Int. J. Cancer 78, 95–99 (1998).
Shou, J. et al. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene 21, 878–889 (2002).
Mazieres, J. et al. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene 24, 5396–5400 (2005).
Hirata, H. et al. Wnt antagonist DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation in human renal cell carcinoma. Int. J. Cancer 128, 1793–1803 (2011).
He, B. et al. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia 6, 7–14 (2004).
MacKeigan, J. P., Murphy, L. O. & Blenis, J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nature Cell Biol. 7, 591–600 (2005).
Choudhury, A. et al. Silencing of ROR1 and FMOD with siRNA results in apoptosis of CLL cells. Br. J. Haematol. 151, 327–335 (2010).
Ueno, K. et al. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br. J. Cancer 101, 1374–1381 (2009).
Ueno, K. et al. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia 10, 697–705 (2008).
Qiang, Y.-W. Wnts induce migration and invasion of myeloma plasma cells. Blood 106, 1786–1793 (2005).
Pukrop, T. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc. Natl Acad. Sci. 103, 5454–5459 (2006).
Ulivieri, A. et al. Frizzled-1 is down-regulated in follicular thyroid tumours and modulates growth and invasiveness. J. Pathol. 215, 87–96 (2008).
Fu, L. et al. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/β-catenin signalling pathway. Gut 60, 1635–1643 (2011).
Yook, J. I. et al. A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nature Cell Biol. 8, 1398–1406 (2006).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Rev. Mol. Cell. Biol. 7, 131–142 (2006).
Etienne-Manneville, S., Manneville, J.-B., Nicholls, S., Ferenczi, M. A. & Hall, A. Cdc42 and Par6-PKCζ regulate the spatially localized association of Dlg1 and APC to control cell polarization. J. Cell Biol. 170, 895–901 (2005).
Schlessinger, K., McManus, E. J. & Hall, A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J. Cell Biol. 178, 355–361 (2007).
Nguyen, D. X. et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138, 51–62 (2009).
Liu, L. et al. Activation of β-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin. Cancer Res. 16, 2740–2750 (2010).
Arozarena, I. et al. In melanoma, β-catenin is a suppressor of invasion. Oncogene 30, 4531–4543 (2011).
Gallagher, S. J. et al. β-catenin inhibits melanocyte migration but induces melanoma metastasis. Oncogene 4 Jun 2012 (doi:10.1038/onc.2012.229).
Damsky, W. E. et al.β-catenin signaling controls metastasis in braf-activated pten-deficient melanomas. Cancer Cell 20, 741–754 (2011).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2012).
Syed Khaja, A. S. et al. Elevated level of Wnt5a protein in localized prostate cancer tissue is associated with better outcome. PLoS ONE 6, e26539 (2011).
Säfholm, A. et al. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J. Biol. Chem. 281, 2740–2749 (2006).
Witze, E. S., Litman, E. S., Argast, G. M., Moon, R. T. & Ahn, N. G. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science 320, 365–369 (2008).
Dow, L. E. et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26, 2272–2282 (2007).
Anastas, J. N. et al. A protein complex of SCRIB, NOS1AP and VANGL1 regulates cell polarity and migration, and is associated with breast cancer progression. Oncogene 31, 3696–3708 (2012).
Lee, J. H. et al. KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Res. 64, 4235–4243 (2004).
Na, H.-W., Shin, W.-S., Ludwig, A. & Lee, S.-T. The cytosolic domain of PTK7, generated from sequential cleavage by ADAM17 and γ-secretase, enhances cell proliferation and migration in colon cancer cells. J. Biol. Chem. 287, 25001–25009 (2012).
Golubkov, V. S. et al. The Wnt/planar cell polarity (PCP) protein tyrosine kinase-7 (PTK7) is a highly efficient proteolytic target of membrane type-1 matrix metalloproteinase (MT1-MMP): implications in cancer and embryogenesis. J. Biol. Chem. 285, 35740–35749 (2010).
Gao, B. et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev. Cell 20, 163–176 (2011).
O'Connell, M. P. et al. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene 29, 34–44 (2010).
Enomoto, M. et al. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene 28, 3197–3208 (2009).
Reifenberger, J. et al. Molecular genetic analysis of malignant melanomas for aberrations of the WNT signaling pathway genes CTNNB1, APC, ICAT and BTRC. Int. J. Cancer 100, 549–556 (2002).
Caldwell, C. M. & Kaplan, K. B. The role of APC in mitosis and in chromosome instability. Adv. Exp. Med. Biol. 656, 51–64 (2009).
Imbert, A., Eelkema, R., Jordan, S., Feiner, H. & Cowin, P. ΔN89β-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J. Cell Biol. 153, 555–568 (2001).
Teissedre, B. et al. MMTV-Wnt1 and -ΔN89β-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS ONE 4, e4537 (2009).
Gounari, F. et al. Stabilization of β-catenin induces lesions reminiscent of prostatic intraepithelial neoplasia, but terminal squamous transdifferentiation of other secretory epithelia. Oncogene 21, 4099–4107 (2002).
Wong, M. H., Rubinfeld, B. & Gordon, J. I. Effects of forced expression of an NH2-terminal truncated β-Catenin on mouse intestinal epithelial homeostasis. J. Cell Biol. 141, 765–777 (1998).
Damalas, A., Kahan, S., Shtutman, M., Ben-Ze'ev, A. & Oren, M. Deregulated β-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 20, 4912–4922 (2001).
Halberg, R. B. et al. Tumorigenesis in the multiple intestinal neoplasia mouse: redundancy of negative regulators and specificity of modifiers. Proc. Natl Acad. Sci. USA 97, 3461–3466 (2000).
Pacheco-Pinedo, E. C. et al. Wnt/ β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J. Clin. Invest. 121, 1935–1945 (2011).
Delmas, V. et al. β-Catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 21, 2923–2935 (2007).
Bulut, G. et al.β-catenin accelerates human papilloma virus type-16 mediated cervical carcinogenesis in transgenic mice. PLoS ONE 6, e27243 (2011).
Smith, M. L., Hawcroft, G. & Hull, M. A. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur. J. Cancer 36, 664–674 (2000).
Dihlmann, S., Siermann, A. & Von Knebel Doeberitz, M. The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate β-catenin/TCF-4 signaling. Oncogene 20, 645–653 (2001).
Tuynman, J. B. et al. Cyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancer. Cancer Res. 68, 1213–1220 (2008).
Labayle, D. et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 101, 635–639 (1991).
Giardiello, F. M. et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 328, 1313–1316 (1993).
Phillips, R. K. S. et al. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 50, 857–860 (2002).
Steinbach, G. et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 342, 1946–1952 (2000).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chem. Biol. 5, 100–107 (2009).
Thorne, C. A. et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nature Chem. Biol. 6, 829–836 (2010).
Huang, S.-M. A. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 (2009).
Gonsalves, F. C. et al. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc. Natl Acad. Sci. USA 108, 5954–5963 (2011).
Wang, W., Liu, H., Wang, S., Hao, X. & Li, L. A diterpenoid derivative 15-oxospiramilactone inhibits Wnt/β-catenin signaling and colon cancer cell tumorigenesis. Cell Res. 21, 730–740 (2011).
Lepourcelet, M. et al. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell 5, 91–102 (2004).
Tian, W. et al. Structure-based discovery of a novel inhibitor targeting the β-catenin/Tcf4 interaction. Biochemistry 51, 724–731 (2012).
Shan, J., Shi, D.-L., Wang, J. & Zheng, J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry 44, 15495–15503 (2005).
Fujii, N. et al. An antagonist of dishevelled protein-protein interaction suppresses β-catenin-dependent tumor cell growth. Cancer Res. 67, 573–579 (2007).
Grandy, D. et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J. Biol. Chem. 284, 16256–16263 (2009).
Zhang, Y. et al. Inhibition of Wnt signaling by Dishevelled PDZ peptides. Nature Chem. Biol. 5, 217–219 (2009).
Nambotin, S. B. et al. Pharmacological inhibition of Frizzled-7 displays anti-tumor properties in hepatocellular carcinoma. J. Hepatol. 54, 288–299 (2011).
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Chen, S. et al. Wnt-1 signaling inhibits apoptosis by activating β-catenin/T cell factor-mediated transcription. J. Cell Biol. 152, 87–96 (2001).
Mikami, I. et al. Efficacy of Wnt-1 monoclonal antibody in sarcoma cells. BMC Cancer 5, 53 (2005).
Pode-Shakked, N. et al. Resistance or sensitivity of Wilms' tumor to anti-FZD7 antibody highlights the Wnt pathway as a possible therapeutic target. Oncogene 30, 1664–1680 (2011).
Ettenberg, S. A. et al. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc. Natl Acad. Sci. USA 107, 15473–15478 (2010). This study finds that antibodies targeting the β-propeller 1 of LRP6 could block WNT1-dependent activation of WNT–CTNNB1 signalling and the growth of MMTV–Wnt1 -driven tumours. By contrast, antibodies targeting the β-propeller 3 of LRP6 could block WNT3A-dependent activation of WNT–CTNNB1 signalling and the growth of MMTV–Wnt3a -driven tumours.These data suggest that different WNTs bind different regions of LRP6 and that different blocking antibodies could be used to target specific WNT signalling pathways.
Hudecek, M. et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood 116, 4532–4541 (2010).
Yang, J. et al. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in B-cell malignancies. PLoS ONE 6, e21018 (2011).
Lavergne, E. et al. Blocking Wnt signaling by SFRP-like molecules inhibits in vivo cell proliferation and tumor growth in cells carrying active β-catenin. Oncogene 30, 423–433 (2011).
Säfholm, A. et al. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin. Cancer Res. 14, 6556–6563 (2008).
DeAlmeida, V. I. et al. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 67, 5371–5379 (2007).
Janda, C. Y., Waghray, D., Levin, A. M., Thomas, C. & Garcia, K. C. Structural basis of Wnt recognition by frizzled. Science 337, 59–64 (2012).
Ohigashi, T., Mizuno, R., Nakashima, J., Marumo, K. & Murai, M. Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells. Prostate 62, 61–68 (2005).
Peng, C., Zhang, X., Yu, H., Wu, D. & Zheng, J. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int. J. Gynecol. Cancer 21, 280–288 (2011).
Zucman-Rossi, J. et al. Differential effects of inactivated Axin1 and activated β-catenin mutations in human hepatocellular carcinomas. Oncogene 26, 774–780 (2007).
Arce, L., Yokoyama, N. N. & Waterman, M. L. Diversity of LEF/TCF action in development and disease. Oncogene 25, 7492–7504 (2006).
Tang, W. et al. A genome-wide RNAi screen for Wnt/ β-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc. Natl Acad. Sci. USA 105, 9697–9702 (2008).
Truica, C. I., Byers, S. & Gelmann, E. P. β-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 60, 4709–4713 (2000).
Pálmer, H. G. et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J. Cell Biol. 154, 369–387 (2001).
Easwaran, V., Pishvaian, M., Salimuddin & Byers, S. Cross-regulation of β-catenin-LEF/TCF and retinoid signaling pathways. Curr. Biol. 9, 1415–1418 (1999).
Yang, F. et al. Linking β-catenin to androgen-signaling pathway. J. Biol. Chem. 277, 11336–11344 (2002).
Shah, S. et al. The molecular basis of vitamin D receptor and β-catenin crossregulation. Mol. Cell 21, 799–809 (2006).
Yang, J. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008).
Medrek, C., Landberg, G., Andersson, T. & Leandersson, K. Wnt-5a-CKIα signaling promotes β-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells. J. Biol. Chem. 284, 10968–10979 (2009).
Qi, L. et al. Dickkopf-1 inhibits epithelial-mesenchymal transition of colon cancer cells and contributes to colon cancer suppression. Cancer Sci. 103, 828–835 (2012).
Ren, D., Minami, Y. & Nishita, M. Critical role of Wnt5a-Ror2 signaling in motility and invasiveness of carcinoma cells following Snail-mediated epithelial-mesenchymal transition. Genes Cells 16, 304–315 (2011).
Djiane, A., Riou, J., Umbhauer, M., Boucaut, J. & Shi, D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 127, 3091–3100 (2000).
Habas, R., Kato, Y. & He, X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843–854 (2001).
Habas, R., Dawid, I. B. & He, X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17, 295–309 (2003).
Wansleeben, C. & Meijlink, F. The planar cell polarity pathway in vertebrate development. Dev. Dyn. 240, 616–626 (2011).
Lai, S.-L., Chien, A. J. & Moon, R. T. Wnt/Fz signaling and the cytoskeleton: potential roles in tumorigenesis. Cell Res. 19, 532–545 (2009).
Hansen, C. et al. Wnt-5a-induced phosphorylation of DARPP-32 inhibits breast cancer cell migration in a CREB-dependent manner. J. Biol. Chem. 284, 27533–27543 (2009).
Acknowledgements
We thank B. Major for his helpful comments on this manuscript. We apologize to our colleagues whose work could not be cited here due to space limitations. R.T.M. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (table)
Summary of literature indicating that WNT signaling proteins may be associated with either favorable or unfavorable outcomes in a pathway-specific, and cancer-subtype-dependent manner. (PDF 340 kb)
Supplementary information S2 (table)
Summary of the molecular targets of novel activators and inhibitors of WNT signaling and the effects of these inhibitors in animals and in cancer model systems (PDF 383 kb)
Glossary
- Constitutively active CTNNB1
-
Various forms of β-catenin (CTNNB1) with either amino-terminal truncations or point mutations that prevent phosphorylation and degradation by the proteasome.
- Feeder cells
-
Additional cells used in co-culture experiments that are intended to support the growth of the cells of interest.
- Basal-like breast cancers
-
A subset of breast cancers that are characterized by a gene expression signature similar to that of the basal and myoepithelial cells of the breast.
- Tumour-initiating cells
-
A subset of cancer cells, often with stem-cell-like expression profiles, that are capable of generating new tumours.
- Mouse mammary tumour virus
-
(MMTV). A species of retrovirus that can drive mammary adenocarcinoma development in susceptible strains of mice in the presence of steroid hormones.
- Scratch assays
-
Assays that are used to determine the motility of cells in vitro. In these experiments, cell monolayers are scratched or wounded and the ability of cells to migrate and fill the resulting gap is measured.
- Planar cell polarity
-
(PCP). The collective orientation of cells within the epithelial plane.
- Apcmin/min mice
-
Mice that are homozygous for the Apcmin allele. Apcmin is a mutant form of Apc that leads to familial adenomatous polyposis (FAP) and spontaneous colorectal tumours.
Rights and permissions
About this article
Cite this article
Anastas, J., Moon, R. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13, 11–26 (2013). https://doi.org/10.1038/nrc3419
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3419
This article is cited by
-
CVD phenotyping in oncologic disorders: cardio-miRNAs as a potential target to improve individual outcomes in revers cardio-oncology
Journal of Translational Medicine (2024)
-
Multifaceted roles for BCL3 in cancer: a proto-oncogene comes of age
Molecular Cancer (2024)
-
Impact of immunohistochemical expression of kinesin family member 18A (Kif18A) and β-catenin in infiltrating breast carcinoma of no special type
World Journal of Surgical Oncology (2024)
-
Targeted activation of ferroptosis in colorectal cancer via LGR4 targeting overcomes acquired drug resistance
Nature Cancer (2024)
-
A host–microbiota interactome reveals extensive transkingdom connectivity
Nature (2024)