Key Points
-
Neuropathic pain syndromes refer to long-lasting 'pathological' states of increased pain intensity (hyperalgesia) or sensitivity (allodynia). These syndromes often result from damage to peripheral nerves, infection or the toxic effects of drugs.
-
Neuropathic pain states are characterized by increased excitability of sensory nociceptive (pain sensing) nerves, changes in the phenotypes of sensory nerves and enhanced synaptic transmission in the spinal cord. However, the cellular and molecular changes that result in these effects are not known. Here, we discuss the possibility that neuroinflammatory responses accompanying neuropathic pain increase the synthesis of molecules that result in pain hypersensitivity.
-
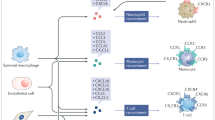
Chemokines (chemotactic cytokines) are small secreted proteins that produce their effects by stimulating G-protein-coupled receptors. There are four families of chemokines and four families of chemokine receptors. Some chemokine receptors, for example, CCR5 and CXCR4, have been shown to be the cellular receptors responsible for HIV-1 infection of target cells. Chemokines have been widely studied owing to their important role in regulating the trafficking of leukocytes under normal conditions and to sites of inflammation. Therefore, chemokines are considered to be major orchestrators of the inflammatory response.
-
Recent work has demonstrated that chemokines and their receptors are also expressed in the nervous system. Chemokines have various effects in the nervous system, ranging from the trafficking of stem cells during embryological development to effects on neuronal excitability and synaptic transmission. In particular, sensory nociceptive neurons express various chemokine receptors. Activation of these receptors by chemokines produces excitation of nociceptors through the transactivation of the TRPV1 (transient receptor potential vanilloid 1) capsaicin receptor, resulting in Na+ influx and neuronal depolarization. Intradermal application of several chemokines produces a strong pain response.
-
In some models of neuropathic pain, the chemokine (CC motif) receptor 2 (CCR2) and its major ligand the chemokine monocyte chemotactic protein-1 (MCP1; also known as CCL2) are upregulated by nociceptive neurons. In association with this, nociceptive neurons in neuropathic pain models are strongly excited by application of MCP1. CCR2-knockout mice show greatly reduced neuropathic pain responses. Therefore, it is possible that direct MCP1 excitation of nociceptive neurons is an important factor in the production of neuropathic pain responses.
-
Drugs that block chemokine receptors, such as CCR2, might constitute a novel class of therapeutic agents for the treatment of painful sensory neuropathies.
Abstract
Chronic (neuropathic) pain is one of the most widespread and intractable of human complaints, as well as being one of the most difficult syndromes to treat successfully with drugs or surgery. The development of new therapeutic approaches to the treatment of painful neuropathies requires a better understanding of the mechanisms that underlie the development of these chronic pain syndromes. It is clear that inflammatory responses often accompany the development of neuropathic pain, and here we discuss the idea that chemokines might be key to integrating the development of pain and inflammation and could furnish new leads in the search for effective analgesic agents for the treatment of painful neuropathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Woolf, C. J. & Mannion, R. J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353, 1959–1964 (1999).
Lewis, W., Day, B. J. & Copeland, W. C. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nature Rev. Drug Discov. 2, 812–822 (2003).
Gonzalez-Scarano, F. & Martin-Garcia, J. The neuropathogenesis of AIDS. Nature Rev. Immunol. 5, 69–81 (2005).
Martin, T. J. & Eisenach, J. C. Pharmacology of opioid and nonopioid analgesics in chronic pain states. J. Pharmacol. Exp. Ther. 299, 811–817 (2001).
Miljanich, G. P. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 11, 3029–3040 (2004).
Frampton, J. E. & Scott, L. J. Pregabalin: in the treatment of painful diabetic peripheral neuropathy. Drugs 64, 2813–2820 (2004).
McClelland, D., Evans, R. M., Barkworth, L., Martin, D. J. & Scott, R. H. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 4, 14 (2004).
Kanai, A., Sarantopoulos, C., McCallum, J. B. & Hogan, Q. Painful neuropathy alters the effect of gabapentin on sensory neuron excitability in rats. Acta Anaesthesiol. Scand. 48, 507–512 (2004).
Passmore, G. M. et al. KCNQ/M currents in sensory neurons: significance for pain therapy. J. Neurosci. 23, 7227–7236 (2003).
Luo, Z. D. et al. Injury type-specific calcium channel a 2 d-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharmacol. Exp. Ther. 303, 1199–1205 (2002).
Bayer, K., Ahmadi, S. & Zeilhofer, H. U. Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology 46, 743–749 (2004).
Ma, C. et al. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J. Neurophysiol. 89, 1588–1602 (2003).
Wu, G. et al. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J. Neurosci. 21, RC140 (2001).
Wu, G. et al. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J. Neurosci. 22, 7746–7753 (2002).
Sukhotinsky, I., Ben-Dor, E., Raber, P. & Devor, M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur. J. Pain 8, 135–143 (2004).
Obata, K. et al. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain 101, 65–77 (2003).
Donovan-Rodriguez, T., Dickenson, A. H. & Urch, C. E. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology 102, 132–140 (2005).
Shimoyama, M., Shimoyama, N. & Hori, Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain 85, 405–414 (2000).
Moore, K. A. et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 22, 6724–6731 (2002).
Sluka, K. A. Blockade of N- and P/Q-type calcium channels reduces the secondary heat hyperalgesia induced by acute inflammation. J. Pharmacol. Exp. Ther. 287, 232–237 (1998).
Lewis, R. J. et al. Novel omega-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J. Biol. Chem. 275, 35335–35344 (2000).
Smith, M. T., Cabot, P. J., Ross, F. B., Robertson, A. D. & Lewis, R. J. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain 96, 119–127 (2002).
Watkins, L. R. & Maier, S. F. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 82, 981–1011 (2002). Excellent review linking the failure of drugs to control neuropathic pain to our ignorance of neuroimmune interactions.
Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 30, 5–11 (1998).
Ransohoff, R. M. The chemokine system in neuroinflammation: an update. J. Infect. Dis. 186 (Suppl. 2), S152–S156 (2002).
Carroll, S. L. & Frohnert, P. W. Expression of JE (monocyte chemoattractant protein-1) is induced by sciatic axotomy in wild type rodents but not in C57BL/Wld(s) mice. J. Neuropathol. Exp. Neurol. 57, 915–930 (1998).
Rutkowski, J. L. et al. Signals for proinflammatory cytokine secretion by human Schwann cells. J. Neuroimmunol. 101, 47–60 (1999).
Siebert, H., Sachse, A., Kuziel, W. A., Maeda, N. & Bruck, W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J. Neuroimmunol. 110, 177–185 (2000).
Perrin, F. E., Lacroix, S., Aviles-Trigueros, M. & David, S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and interleukin-1b in Wallerian degeneration. Brain 128, 854–866 (2005).
Meller, S. T., Dykstra, C., Grzybycki, D., Murphy, S. & Gebhart, G. F. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology 33, 1471–1478 (1994).
DeLeo, J. A. & Yezierski, R. P. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90, 1–6 (2001).
Cunha, F. Q., Poole, S., Lorenzetti, B. B. & Ferreira, S. H. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 107, 660–664 (1992). Established that proinflammatory cytokines have an early and crucial role in the development of inflammatory hyperalgesia.
Safieh-Garabedian, B., Poole, S., Allchorne, A., Winter, J. & Woolf, C. J. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 115, 1265–1275 (1995).
Ohtori, S., Takahashi, K., Moriya, H. & Myers, R. R. TNF-α and TNF-α receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine 29, 1082–1088 (2004).
Holmes, G. M., Hebert, S. L., Rogers, R. C. & Hermann, G. E. Immunocytochemical localization of TNF type 1 and type 2 receptors in the rat spinal cord. Brain Res. 1025, 210–219 (2004).
Shubayev, V. I. & Myers, R. R. Upregulation and interaction of TNFα and gelatinases A and B in painful peripheral nerve injury. Brain Res. 855, 83–89 (2000).
Schafers, M., Geis, C., Svensson, C. I., Luo, Z. D. & Sommer, C. Selective increase of tumour necrosis factor-α in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur. J. Neurosci. 17, 791–804 (2003).
Sommer, C. Painful neuropathies. Curr. Opin. Neurol. 16, 623–628 (2004).
Schafers, M., Lee, D. H., Brors, D., Yaksh, T. L. & Sorkin, L. S. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-α after spinal nerve ligation. J. Neurosci. 23, 3028–3038 (2003).
Schafers, M., Svensson, C. I., Sommer, C. & Sorkin, L. S. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 23, 2517–2521 (2003).
Liu, B., Li, H., Brull, S. J. & Zhang, J. -M. Increased sensitivity of sensory neurons to tumor necrosis factor a in rats with chronic compression of the lumbar ganglia. J. Neurophysiol. 88, 1393–1399 (2002).
Subang, M. C. & Richardson, P. M. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur. J. Neurosci. 13, 521–528 (2001).
Tanaka, T., Minami, M., Nakagawa, T. & Satoh, M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci. Res. 48, 463–469 (2004).
White, F. A. et al. MCP-1/CCR2 signaling is upregulated in a subset of sensory neurons subjected to chronic compression injury. Proc. Natl Acad. Sci. USA (in the press).
Lindenlaub, T., Teuteberg, P., Hartung, T. & Sommer, C. Effects of neutralizing antibodies to TNF-α on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res. 866, 15–22 (2000).
Sommer, C. et al. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 913, 86–89 (2001).
Raghavendra, V., Tanga, F. & DeLeo, J. A. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 306, 624–630 (2003).
Abbadie, C. et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl Acad. Sci. USA 100, 7947–7952 (2003). Showed that genetic deletion of CCR2 diminishes injury-induced neuropathic pain.
Oh, S. B., Endoh, T., Simen, A. A., Ren, D. & Miller, R. J. Regulation of calcium currents by chemokines and their receptors. J. Neuroimmunol. 123, 66–75 (2002).
Oh, S. B. et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 21, 5027–5035 (2001). The first paper describing the expression of diverse chemokine receptors by DRG neurons and their potential role in the generation of pain.
Qin, X., Wan, Y. & Wang, X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J. Neurosci. Res. 26 July 2005 (10.1002/jnr.20612).
Rossi, D. & Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18, 217–242 (2000).
Tran, P. B. & Miller, R. J. Chemokine receptors: signposts to brain development and disease. Nature Rev. Neurosci. 4, 444–455 (2003).
Rot, A. & von Andrian, U. H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 22, 891–928 (2004).
Cartier, L., Hartley, O., Dubois-Dauphin, M. & Krause, K. H. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res. Brain Res. Rev. 48, 16–42 (2005).
Zou, Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998). Noteworthy as the first report of a role for a chemokine receptor in neuronal cell migration and development of the CNS.
Lu, M., Grove, E. A. & Miller, R. J. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl Acad. Sci. USA 99, 7090–7095 (2002).
Stumm, R. K. et al. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J. Neurosci. 22, 5865–5878 (2002).
Banisadr, G., Skrzydelski, D., Kitabgi, P., Rostene, W. & Parsadaniantz, S. M. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur. J. Neurosci. 18, 1593–1606 (2003).
Banisadr, G. et al. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur. J. Neurosci. 16, 1661–1671 (2002).
Banisadr, G. et al. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J. Neurochem. 81, 257–269 (2002).
Cowell, R. M. & Silverstein, F. S. Developmental changes in the expression of chemokine receptor CCR1 in the rat cerebellum. J. Comp. Neurol. 457, 7–23 (2003).
Tissir, F., Wang, C. E. & Goffinet, A. M. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res. Dev. Brain Res. 149, 63–71 (2004).
Ragozzino, D. CXC chemokine receptors in the central nervous system: role in cerebellar neuromodulation and development. J. Neurovirol. 8, 559–572 (2002).
Nelson, T. E. & Gruol, D. L. The chemokine CXCL10 modulates excitatory activity and intracellular calcium signaling in cultured hippocampal neurons. J. Neuroimmunol. 156, 74–87 (2004).
Puma, C., Danik, M., Quirion, R., Ramon, F. & Williams, S. The chemokine interleukin-8 acutely reduces Ca2+ currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J. Neurochem. 78, 960–971 (2001).
Zhang, N. et al. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc. Natl Acad. Sci. USA 102, 4536–4541 (2005). Showed that a chemokine acting on its cognate chemokine receptor can cross-sensitize TRPV1 and contribute to hyperalgesia during inflammation.
Verge, G. M. et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur. J. Neurosci. 20, 1150–1160 (2004). Provides evidence that the presence of chemokine receptors on spinal microglia is associated with nociceptive transmission and potentially neuropathic pain mechanisms.
Bolin, L. M. et al. Primary sensory neurons migrate in response to the chemokine RANTES. J. Neuroimmunol. 81, 49–57 (1998). Suggests that the presence of the chemokine RANTES is essential for neuronal migration and differentiation of nociceptive neurons in the DRG.
Homma, Y., Brull, S. J. & Zhang, J. M. A comparison of chronic pain behavior following local application of tumor necrosis factor a to the normal and mechanically compressed lumbar ganglia in the rat. Pain 95, 239–246 (2002).
Song, X. J., Hu, S. J., Greenquist, K. W., Zhang, J. M. & LaMotte, R. H. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J. Neurophysiol. 82, 3347–3358 (1999). Showed that chronic compression of the ganglia produces enhanced excitability in the DRG.
Zhang, J. M., Song, X. J. & LaMotte, R. H. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J. Neurophysiol. 82, 3359–3366 (1999).
Hu, S. -J., Song, X. -J., Greenquist, K. W., Zhang, J. -M. & LaMotte, R. H. Protein kinase A modulates spontaneous activity in chronically compressed dorsal root ganglion neurons in the rat. Pain 94, 39–46 (2001).
Tofaris, G. K., Patterson, P. H., Jessen, K. R. & Mirsky, R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J. Neurosci. 22, 6696–6703 (2002).
Glabinski, A. R. et al. TNF-α microinjection upregulates chemokines and chemokine receptors in the central nervous system without inducing leukocyte infiltration. J. Interferon Cytokine Res. 23, 457–466 (2003).
Belmadani, A. et al. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J. Neurosci. 25, 3995–4003 (2005).
Fujioka, T., Purev, E. & Rostami, A. Chemokine mRNA expression in the cauda equina of Lewis rats with experimental allergic neuritis. J. Neuroimmunol. 97, 51–59 (1999).
Sorensen, T. L. et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 103, 807–815 (1999).
Milligan, E. D. et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur. J. Neurosci. 20, 2294–2302 (2004).
Lindia, J. A., McGowan, E., Jochnowitz, N. & Abbadie, C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J. Pain 6, 434–438 (2005).
Garton, K. J. et al. Tumor necrosis factor-α-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J. Biol. Chem. 276, 37993–38001 (2001).
Tsou, C. L., Haskell, C. A. & Charo, I. F. Tumor necrosis factor-a-converting enzyme mediates the inducible cleavage of fractalkine. J. Biol. Chem. 276, 44622–44626 (2001).
Ludwig, A., Berkhout, T., Moores, K., Groot, P. & Chapman, G. Fractalkine is expressed by smooth muscle cells in response to IFN-gamma and TNF-α and is modulated by metalloproteinase activity. J. Immunol. 168, 604–612 (2002).
Johnston, I. N. et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 24, 7353–7365 (2004).
Marzocchetti, A. et al. Macrophage chemoattractant protein-1 levels in cerebrospinal fluid correlate with containment of JC virus and prognosis of acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. J. Neurovirol. 11, 219–224 (2005).
Avison, M. J. et al. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J. Neurovirol. 10, 223–232 (2004).
Sodhi, A., Montaner, S. & Gutkind, J. S. Viral hijacking of G-protein-coupled-receptor signalling networks. Nature Rev. Mol. Cell Biol. 5, 998–1012 (2004).
Zhu, Y. et al. Lentivirus infection causes neuroinflammation and neuronal injury in dorsal root ganglia: pathogenic effects of STAT-1 and inducible nitric oxide synthase. J. Immunol. 175, 1118–1126 (2005).
Keswani, S. C. et al. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann. Neurol. 54, 287–296 (2003).
Milligan, E. D. et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 861, 105–116 (2000).
Hall, C. D. et al. Peripheral neuropathy in a cohort of human immunodeficiency virus-infected patients. Incidence and relationship to other nervous system dysfunction. Arch. Neurol. 48, 1273–1274 (1991).
Bacellar, H. et al. Temporal trends in the incidence of HIV-1-related neurologic diseases: multicenter AIDS cohort study, 1985–1992. Neurology 44, 1892–1900 (1994).
Snider, W. D. et al. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann. Neurol. 14, 403–418 (1983). First observation of painful peripheral neuropathies in HIV-positive individuals.
Lee, B. J., Koszinowski, U. H., Sarawar, S. R. & Adler, H. A g-herpesvirus G protein-coupled receptor homologue is required for increased viral replication in response to chemokines and efficient reactivation from latency. J. Immunol. 170, 243–251 (2003).
Bodner, A. et al. Mixed lineage kinase 3 mediates gp120IIIB-induced neurotoxicity. J. Neurochem. 82, 1424–1434 (2002).
Ho, D. D. et al. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N. Engl. J. Med. 313, 1493–1497 (1985).
Kaul, M., Garden, G. A. & Lipton, S. A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 (2001).
An, S. F., Groves, M., Gray, F. & Scaravilli, F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J. Neuropathol. Exp. Neurol. 58, 1156–1162 (1999).
Keswani, S. C. et al. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann. Neurol. 53, 57–64 (2003).
Cherry, C. L., McArthur, J. C., Hoy, J. F. & Wesselingh, S. L. Nucleoside analogues and neuropathy in the era of HAART. J. Clin. Virol. 26, 195–207 (2003).
Dalakas, M. C. Peripheral neuropathy and antiretroviral drugs. J. Peripher. Nerv. Syst. 6, 14–20 (2001).
Kakuda, T. N. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 22, 685–708 (2000).
Keilbaugh, S. A., Hobbs, G. A. & Simpson, M. V. Effect of 2′,3′-dideoxycytidine on oxidative phosphorylation in the PC12 cell, a neuronal model. Biochem. Pharmacol. 53, 1485–1492 (1997).
Joseph, E. K., Chen, X., Khasar, S. G. & Levine, J. D. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain 107, 147–158 (2004).
Aley, K. O. & Levine, J. D. Different peripheral mechanisms mediate enhanced nociception in metabolic/toxic and traumatic painful peripheral neuropathies in the rat. Neuroscience 111, 389–397 (2002).
Aley, K. O., McCarter, G. & Levine, J. D. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J. Neurosci. 18, 7008–7014 (1998).
Dina, O. A. et al. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J. Neurosci. 20, 8614–8619 (2000).
Schmued, L. C. et al. Evaluation of brain and nerve pathology in rats chronically dosed with ddI or isoniazid. Neurotoxicol. Teratol. 18, 555–563 (1996).
Tyor, W. R., Wesselingh, S. L., Griffin, J. W., McArthur, J. C. & Griffin, D. E. Unifying hypothesis for the pathogenesis of HIV-associated dementia complex, vacuolar myelopathy, and sensory neuropathy. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9, 379–388 (1995).
Wulff, E. A., Wang, A. K. & Simpson, D. M. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs 59, 1251–1260 (2000).
Norton, G. R., Sweeney, J., Marriott, D., Law, M. G. & Brew, B. J. Association between HIV distal symmetric polyneuropathy and Mycobacterium avium complex infection. J. Neurol. Neurosurg. Psychiatry 61, 606–609 (1996).
Herzberg, U. & Sagen, J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J. Neuroimmunol. 116, 29–39 (2001).
Milligan, E. D. et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 21, 2808–2819 (2001).
Foley, J. F. et al. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte-derived macrophages, dendritic cells, and lymph nodes. J. Immunol. 174, 4892–4900 (2005).
Ma, Q. -P. The expression of bradykinin B1 receptors on primary sensory neurones that give rise to small caliber sciatic nerve fibres in rats. Neuroscience 107, 665–673 (2001).
Ma, Q. P., Hill, R. & Sirinathsinghji, D. Basal expression of bradykinin B1 receptor in peripheral sensory ganglia in the rat. Neuroreport 11, 4003–4005 (2000).
Wotherspoon, G. & Winter, J. Bradykinin B1 receptor is constitutively expressed in the rat sensory nervous system. Neurosci. Lett. 294, 175–178 (2000).
Davis, C. L. et al. B1 bradykinin receptors and sensory neurones. Br. J. Pharmacol. 118, 1469–1476 (1996).
Davis, A. J. & Perkins, M. N. Substance P and capsaicin-induced mechanical hyperalgesia in the rat knee joint; the involvement of bradykinin B1 and B2 receptors. Br. J. Pharmacol. 118, 2206–2212 (1996).
Julius, D. & Basbaum, A. I. Molecular mechanisms of nociception. Nature 413, 203–210 (2001).
Chuang, H. -H. et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411, 957–962 (2001).
Grimm, M. C. et al. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J. Exp. Med. 188, 317–325 (1998). Showed that opioids could directly interact with chemokine receptors, thereby effectively desensitizing them.
Zhang, N., Rogers, T. J., Caterina, M. & Oppenheim, J. J. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J. Immunol. 173, 594–599 (2004). Describes the observation that proinflammatory chemokines could interact with opioid receptors, thereby effectively desensitizing the neuronal receptors.
Rogers, T. J. & Peterson, P. K. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 24, 116–121 (2003).
Chen, C. et al. Heterodimerization and cross-desensitization between the m-opioid receptor and the chemokine CCR5 receptor. Eur. J. Pharmacol. 483, 175–186 (2004).
Suzuki, S., Chuang, L. F., Yau, P., Doi, R. H. & Chuang, R. Y. Interactions of opioid and chemokine receptors: oligomerization of m, k, and d with CCR5 on immune cells. Exp. Cell Res. 280, 192–200 (2002).
Toth, P. T., Ren, D. & Miller, R. J. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1α and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J. Pharmacol. Exp. Ther. 310, 8–17 (2004).
Szabo, I. et al. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J. Leukoc. Biol. 74, 1074–1082 (2003).
Raghavendra, V., Rutkowski, M. D. & DeLeo, J. A. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci. 22, 9980–9989 (2002).
Brack, A. et al. Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain 112, 229–238 (2004).
Mousa, S. A. Morphological correlates of immune-mediated peripheral opioid analgesia. Adv. Exp. Med. Biol. 521, 77–87 (2003).
Neilan, C. L. et al. Experimental neuropathic pain in mice. Soc. Neurosci. Abstr. 17.4 (2004).
Horuk, R. Development and evaluation of pharmacological agents targeting chemokine receptors. Methods 29, 369–375 (2003).
De Clercq, E. The bicyclam AMD3100 story. Nature Rev. Drug Discov. 2, 581 (2003).
Onuffer, J. J. & Horuk, R. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol. Sci. 23, 459–467 (2002).
Daly, C. & Rollins, B. J. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation 10, 247–257 (2003).
Bertini, R. et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc. Natl Acad. Sci. USA 101, 11791–11796 (2004).
Ribeiro, S. & Horuk, R. The clinical potential of chemokine receptor antagonists. Pharmacol. Ther. 107, 44–58 (2005).
Luger, N. M., Mach, D. B., Sevcik, M. A. & Mantyh, P. W. Bone cancer pain: from model to mechanism to therapy. J. Pain Symptom Manage. 29, S32–S46 (2005).
Polomano, R. C., Mannes, A. J., Clark, U. S. & Bennett, G. J. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94, 293–304 (2001).
Calcutt, N. A. Experimental models of painful diabetic neuropathy. J. Neurol. Sci. 220, 137–139 (2004).
Mills, C. D., Hains, B. C., Johnson, K. M. & Hulsebosch, C. E. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J. Neurotrauma 18, 743–756 (2001).
Aicher, S. A., Silverman, M. B., Winkler, C. W. & Bebo, B. F. Jr. Hyperalgesia in an animal model of multiple sclerosis. Pain 110, 560–570 (2004).
Wallace, V. C. J., Cottrell, D. F., Brophy, P. J. & Fleetwood-Walker, S. M. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J. Neurosci. 23, 3221–3233 (2003).
Kury, P., Greiner-Petter, R., Cornely, C., Jurgens, T. & Muller, H. W. Mammalian achaete scute homolog 2 is expressed in the adult sciatic nerve and regulates the expression of Krox24, Mob-1, CXCR4, and p57kip2 in Schwann cells. J. Neurosci. 22, 7586–7595 (2002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- GLIAL FIBRILLARY ACIDIC PROTEIN
-
(GFAP). Principle astrocyte intermediate filament that is upregulated in Schwann cells following injury. It is likely to play a direct role in the subsequent inflammatory response.
- NOCICEPTORS
-
Sensory neurons that respond to pain and noxious stimulation.
- PARATHESIAS
-
Abnormal or unpleasant sensations that result from injury to one or more nerves, often described by patients as numbness or as prickly, stinging or burning feelings.
- MYELINOPATHY
-
The degeneration of myelin sheaths of neurons.
- BRADYKININ
-
Small peptide of the kinin family that excites peripheral nerves and regulates the contraction of blood vessels and fluid transport by epithelia.
Rights and permissions
About this article
Cite this article
White, F., Bhangoo, S. & Miller, R. Chemokines: Integrators of Pain and Inflammation. Nat Rev Drug Discov 4, 834–844 (2005). https://doi.org/10.1038/nrd1852
Issue Date:
DOI: https://doi.org/10.1038/nrd1852
This article is cited by
-
Modulatory Effects of Stem Cells on Opioid Receptors and Neuroinflammation
Current Pain and Headache Reports (2022)
-
Dynamic monocyte chemoattractant protein-1 level as predictors of perceived pain during first and second phacoemulsification eye surgeries in patients with bilateral cataract
BMC Ophthalmology (2021)
-
Paeoniflorin attenuates chronic constriction injury-induced neuropathic pain by suppressing spinal NLRP3 inflammasome activation
Inflammopharmacology (2020)
-
Neuromodulation, Specialized Proresolving Mediators, and Resolution of Pain
Neurotherapeutics (2020)
-
Pain and immunity: implications for host defence
Nature Reviews Immunology (2019)