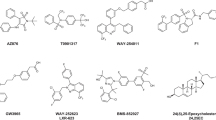
Key Points
-
The liver X receptors (LXRs) are sterol-sensitive transcription factors that regulate cholesterol homeostasis.
-
LXRs control the expression of genes that are linked to lipid synthesis, transport and excretion in many tissues.
-
LXRs are crucial regulators of the reverse cholesterol transport pathway and are important determinants of whole-body cholesterol content.
-
Pharmacological activation of LXRs inhibits the development of atherosclerosis in animal models.
-
Subtype-selective LXR agonists and tissue-selective agonists are promising strategies for the development of targeted modulators of lipid metabolism.
-
Alterations in LXR-dependent gene expression and cholesterol metabolism have been associated with the development of neurological diseases, including Alzheimer's disease.
-
LXR is a promising therapeutic target but the development of novel drugs faces many challenges, including undesirable hepatic side effects.
Abstract
The liver X receptors (LXRs) are pivotal regulators of lipid homeostasis in mammals. These transcription factors control the expression of a battery of genes involved in the uptake, transport, efflux and excretion of cholesterol in a tissue-dependent manner. The identification of the LXRs, and an increased understanding of the mechanisms by which LXR signalling regulates lipid homeostasis in different tissues (including the liver, intestine and brain), has highlighted new opportunities for therapeutic intervention in human metabolism. New strategies for the pharmacological manipulation of LXRs and their target genes offer promise for the treatment of human diseases in which lipids have a central role, including atherosclerosis and Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brown, M. S. & Goldstein, J. L. A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 (1986).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Peet, D. J., Janowski, B. A. & Mangelsdorf, D. J. The LXRs: a new class of oxysterol receptors. Curr. Opin. Genet. Dev. 8, 571–575 (1998).
Peet, D. J. et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 93, 693–704 (1998). This paper describes the Lxra -knockout mouse and characterizes that it has a defect in CYP7A expression, providing direct evidence that LXRα has a role in cholesterol metabolism.
Collins, J. L. et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J. Med. Chem. 45, 1963–1966 (2002).
Yang, C. et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 281, 27816–27826 (2006).
Frolov, A. et al. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. J. Biol. Chem. 278, 25517–25525 (2003).
Fu, X. et al. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 276, 38378–38387 (2001).
Spencer, T. A. et al. 24(S),25-epoxycholesterol. Evidence consistent with a role in the regulation of hepatic cholesterogenesis. J. Biol. Chem. 260, 13391–13394 (1985).
Svensson, S. et al. Crystal structure of the heterodimeric complex of LXRα and RXRβ ligand-binding domains in a fully agonistic conformation. EMBO J. 22, 4625–4633 (2003). This paper reports the crystal structure of the LXR–RXR heterodimer.
Wagner, B. L. et al. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol. Cell. Biol. 23, 5780–5789 (2003).
Hu, X., Li, S., Wu, J., Xia, C. & Lala, D. S. Liver X receptors interact with corepressors to regulate gene expression. Mol. Endocrinol. 17, 1019–1026 (2003).
Huuskonen, J., Fielding, P. E. & Fielding, C. J. Role of p160 coactivator complex in the activation of liver X receptor. Arterioscler. Thromb. Vasc. Biol. 24, 703–708 (2004).
Lee, S., Lee, J., Lee, S. K. & Lee, J. W. Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol. Endocrinol. 22, 1312–1319 (2008).
Kim, G. H. et al. Characterization of ASC-2 as an antiatherogenic transcriptional coactivator of liver X receptors in macrophages. Mol. Endocrinol. 23, 966–974 (2009).
Son, Y. L. & Lee, Y. C. Molecular determinants of the interactions between LXR/RXR heterodimers and TRAP220. Biochem. Biophys. Res. Commun. 384, 389–393 (2009).
Repa, J. J. et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289, 1524–1529 (2000). This paper shows that RXR heterodimers are powerful regulators of intestinal cholesterol absorption.
Boergesen, M. et al. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor α in mouse liver reveals extensive sharing of binding sites. Mol. Cell. Biol. 32, 852–867 (2012).
Quack, M., Frank, C. & Carlberg, C. Differential nuclear receptor signalling from DR4-type response elements. J. Cell Biochem. 86, 601–612 (2002).
Gabbi, C., Warner, M. & Gustafsson, J. K. Action mechanisms of liver X receptors. Biochem. Biophys. Res. Commun. http://dx.doi.org/10.1016/j.bbrc.2013.11.077 (2013).
Kidani, Y. & Bensinger, S. J. Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol. Rev. 249, 72–83 (2012).
Laffitte, B. A. et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl Acad. Sci. USA 100, 5419–5424 (2003).
Laffitte, B. A. et al. The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions. Mol. Cell. Biol. 23, 2182–2191 (2003).
Laffitte, B. A. et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl Acad. Sci. USA 98, 507–512 (2001).
Venkateswaran, A. et al. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J. Biol. Chem. 275, 14700–14707 (2000).
Repa, J. J. et al. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors α and β. J. Biol. Chem. 277, 18793–18800 (2002).
Repa, J. J. et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes Dev. 14, 2819–2830 (2000). This paper identifies the key lipogenic transcription factor SREBP1C as an LXR target gene, thereby linking LXR activation with fatty acid metabolism.
Bradley, M. N. et al. Ligand activation of LXRβ reverses atherosclerosis and cellular cholesterol overload in mice lacking LXRα and apoE. J. Clin. Invest. 117, 2337–2346 (2007).
Joseph, S. B., Castrillo, A., Laffitte, B. A., Mangelsdorf, D. J. & Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature Med. 9, 213–219 (2003).
Joseph, S. B. et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl Acad. Sci. USA 99, 7604–7609 (2002). This paper provides the first demonstration that pharmacological activation of LXR could inhibit the development of atherosclerosis.
Tangirala, R. K. et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl Acad. Sci. USA 99, 11896–11901 (2002).
Wang, N. & Tall, A. R. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23, 1178–1184 (2003).
Tarling, E. J. & Edwards, P. A. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl Acad. Sci. USA 108, 19719–19724 (2011).
Brooks-Wilson, A. et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nature Genet. 22, 336–345 (1999).
Bodzioch, M. et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nature Genet. 22, 347–351 (1999).
Rust, S. et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nature Genet. 22, 352–355 (1999).
Brunham, L. R. et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116, 1052–1062 (2006).
Brunham, L. R. et al. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ. Res. 99, 672–674 (2006).
Hong, C. et al. Constitutive activation of LXR in macrophages regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target. J. Lipid Res. 52, 531–539 (2011).
Mak, P. A. et al. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors α and β. J. Biol. Chem. 277, 31900–31908 (2002).
Hummasti, S. et al. Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of apoD as a direct target. J. Lipid Res. 45, 616–625 (2004).
Zhang, Y., Repa, J. J., Gauthier, K. & Mangelsdorf, D. J. Regulation of lipoprotein lipase by the oxysterol receptors, LXRα and LXRβ. J. Biol. Chem. 276, 43018–43024 (2001).
Luo, Y. & Tall, A. R. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J. Clin. Invest. 105, 513–520 (2000).
Honzumi, S. et al. LXRα regulates human CETP expression in vitro and in transgenic mice. Atherosclerosis 212, 139–145 (2010).
Hong, C. et al. LXRα is uniquely required for maximal reverse cholesterol transport and atheroprotection in ApoE-deficient mice. J. Lipid Res. 53, 1126–1133 (2012).
Bischoff, E. D. et al. Non-redundant roles for LXRα and LXRβ in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J. Lipid Res. 51, 900–906 (2010).
Zhang, Y. et al. Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J. Clin. Invest. 122, 1688–1699 (2012).
Nanjee, M. N., Doran, J. E., Lerch, P. G. & Miller, N. E. Acute effects of intravenous infusion of ApoA1/phosphatidylcholine discs on plasma lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 19, 979–989 (1999).
Stein, O. & Stein, Y. The removal of cholesterol from Landschutz ascites cells by high-density apolipoprotein. Biochim. Biophys. Acta 326, 232–244 (1973).
Naik, S. U. et al. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation 113, 90–97 (2006).
Zanotti, I. et al. The LXR agonist T0901317 promotes the reverse cholesterol transport from macrophages by increasing plasma efflux potential. J. Lipid Res. 49, 954–960 (2008).
Joseph, S. B. et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 277, 11019–11025 (2002).
Chu, K., Miyazaki, M., Man, W. C. & Ntambi, J. M. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol. Cell. Biol. 26, 6786–6798 (2006).
Kiss, E. et al. Suppression of chronic damage in renal allografts by liver X receptor (LXR) activation relevant contribution of macrophage LXRα. Am. J. Pathol. 179, 92–103 (2011).
Willy, P. J. et al. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9, 1033–1045 (1995).
Beaven, S. W. et al. Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell. Metab. 18, 106–117 (2013).
Kratzer, A. et al. Synthetic LXR agonist attenuates plaque formation in apoE−/− mice without inducing liver steatosis and hypertriglyceridemia. J. Lipid Res. 50, 312–326 (2009).
Claudel, T. et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc. Natl Acad. Sci. USA 98, 2610–2615 (2001).
Teupser, D. et al. Effect of macrophage overexpression of murine liver X receptor-α (LXR-α) on atherosclerosis in LDL-receptor deficient mice. Arterioscler. Thromb. Vasc. Biol. 28, 2009–2015 (2008).
Zhao, Y. et al. Enhanced foam cell formation, atherosclerotic lesion development, and inflammation by combined deletion of ABCA1 and SR-BI in bone marrow-derived cells in LDL receptor knockout mice on western-type diet. Circ. Res. 107, e20–e31 (2010).
Miao, B. et al. Raising HDL cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective LXR modulator. J. Lipid Res. 45, 1410–1417 (2004).
Peng, D. et al. Antiatherosclerotic effects of a novel synthetic tissue-selective steroidal liver X receptor agonist in low-density lipoprotein receptor-deficient mice. J. Pharmacol. Exp. Ther. 327, 332–342 (2008).
Fowler, A. J. et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J. Invest. Dermatol. 120, 246–255 (2003).
Marathe, C. et al. The arginase II gene is an anti-inflammatory target of liver X receptor in macrophages. J. Biol. Chem. 281, 32197–32206 (2006).
Castrillo, A. et al. Crosstalk between LXR and Toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell 12, 805–816 (2003).
Castrillo, A., Joseph, S. B., Marathe, C., Mangelsdorf, D. J. & Tontonoz, P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J. Biol. Chem. 278, 10443–10449 (2003).
Feig, J. E. et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Invest. 120, 4415–4424 (2010).
Maxwell, K. N. & Breslow, J. L. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl Acad. Sci. USA 101, 7100–7105 (2004).
Zelcer, N., Hong, C., Boyadjian, R. & Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 325, 100–104 (2009). This paper identifies the LXR-regulated E3 ligase IDOL as an SREBP-independent mechanism for feedback inhibition of cholesterol uptake.
Chang, Y. S. et al. Stapled α-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl Acad. Sci. USA 110, E3445–E3454 (2013).
Leao, M. et al. Discovery of a new small-molecule inhibitor of p53-MDM2 interaction using a yeast-based approach. Biochem. Pharmacol. 85, 1234–1245 (2013).
Paiva, A. M. et al. Development of noncytotoxic PLGA nanoparticles to improve the effect of a new inhibitor of p53-MDM2 interaction. Int. J. Pharm. 454, 394–402 (2013).
Sorrentino, V. et al. Distinct functional domains contribute to degradation of the low density lipoprotein receptor (LDLR) by the E3 ubiquitin ligase inducible degrader of the LDLR (IDOL). J. Biol. Chem. 286, 30190–30199 (2011).
Calkin, A. C. et al. FERM-dependent E3 ligase recognition is a conserved mechanism for targeted degradation of lipoprotein receptors. Proc. Natl Acad. Sci. USA 108, 20107–20112 (2011).
Zhang, L. et al. The IDOL–UBE2D complex mediates sterol-dependent degradation of the LDL receptor. Genes Dev. 25, 1262–1274 (2011).
Scotti, E. et al. IDOL stimulates clathrin-independent endocytosis and multivesicular body-mediated lysosomal degradation of the low-density lipoprotein receptor. Mol. Cell. Biol. 33, 1503–1514 (2013).
Sorrentino, V. et al. The LXR–IDOL axis defines a clathrin, caveolae, and dynamin-independent endocytic route for LDLR internalization and lysosomal degradation. J. Lipid Res. 54, 2174–2184 (2013).
El Hajj, H. I. et al. The inflammatory response in acyl-CoA oxidase 1 deficiency (pseudoneonatal adrenoleukodystrophy). Endocrinology 153, 2568–2575 (2012).
Scotti, E. et al. Targeted disruption of the idol gene alters cellular regulation of the low-density lipoprotein receptor by sterols and liver X receptor agonists. Mol. Cell. Biol. 31, 1885–1893 (2011).
Weissglas-Volkov, D. et al. The N342S MYLIP polymorphism is associated with high total cholesterol and increased LDL receptor degradation in humans. J. Clin. Invest. 121, 3062–3071 (2011).
Chasman, D. I. et al. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ. Cardiovasc. Genet. 5, 257–264 (2012).
Teslovitch, T. M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 (2010).
Sorrentino, V. et al. Identification of a loss-of-function inducible degrader of the low-density lipoprotein receptor variant in individuals with low circulating low-density lipoprotein. Eur. Heart J. 34, 1292–1297 (2013).
Temel, R. E. et al. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell. Metab. 12, 96–102 (2010).
Brown, J. M. et al. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J. Biol. Chem. 283, 10522–10534 (2008).
Temel, R. E. & Brown, J. M. Biliary and nonbiliary contributions to reverse cholesterol transport. Curr. Opin. Lipidol 23, 85–90 (2012).
Yu, L. et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl Acad. Sci. USA 99, 16237–16242 (2002). This paper defines the importance of the transporters ABCG5 and ABCG8 in controlling sterol excretion and maintaining cholesterol homeostasis in mice.
Altmann, S. W. et al. Niemann–Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Davis, H. R. Jr et al. Niemann–Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279, 33586–33592 (2004).
Kruit, J. K. et al. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology 128, 147–156 (2005).
van der Veen, J. N. et al. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284, 19211–19219 (2009).
Venkateswaran, A. et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα. Proc. Natl Acad. Sci. USA 97, 12097–12102 (2000).
Plosch, T. et al. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J. Biol. Chem. 277, 33870–33877 (2002).
Timmins, J. M. et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115, 1333–1342 (2005).
Yu, L. et al. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol. Chem. 278, 15565–15570 (2003).
Berge, K. E. et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290, 1771–1775 (2000).
Yu, L. et al. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110, 671–680 (2002).
Lo Sasso, G. et al. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell. Metab. 12, 187–193 (2010).
Yasuda, T. et al. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 30, 781–786 (2010). This paper describes the first intestine-specific LXR agonist and provides proof of concept for tissue-selective LXR activation strategies.
Theofilopoulos, S. et al. Brain endogenous liver X receptor ligands selectively promote midbrain neurogenesis. Nature Chem. Biol. 9, 126–133 (2013).
Hogenboom, S. et al. Phosphomevalonate kinase is a cytosolic protein in humans. J. Lipid Res. 45, 697–705 (2004).
Frenkel, J. et al. Lack of isoprenoid products raises ex vivo interleukin-1β secretion in hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis Rheum. 46, 2794–2803 (2002).
Ebberink, M. S. et al. Genetic classification and mutational spectrum of more than 600 patients with a Zellweger syndrome spectrum disorder. Hum. Mutat. 32, 59–69 (2011).
Orth, M. & Bellosta, S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol 2012, 292598 (2012).
Van Hove, J. L. et al. Succinyl-CoA ligase deficiency: a mitochondrial hepatoencephalomyopathy. Pediatr. Res. 68, 159–164 (2010).
Wanders, R. J. & Waterham, H. R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 75, 295–332 (2006).
Zelcer, N. et al. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver X receptors. Proc. Natl Acad. Sci. USA 104, 10601–10606 (2007).
Jiang, Q. et al. ApoE promotes the proteolytic degradation of Aβ. Neuron 58, 681–693 (2008). This paper shows that the ability of LXR to lipidate APOE by increasing ABCA1 activity is an important determinant of amyloid-β clearance and of the induction of Alzheimer's disease-like pathology in mice.
Morales, J. R. et al. Activation of liver X receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation 118, 1450–1459 (2008).
Sironi, L. et al. Treatment with LXR agonists after focal cerebral ischemia prevents brain damage. FEBS Lett. 582, 3396–3400 (2008).
Fan, X., Kim, H. J., Bouton, D., Warner, M. & Gustafsson, J. A. Expression of liver X receptor β is essential for formation of superficial cortical layers and migration of later-born neurons. Proc. Natl Acad. Sci. USA 105, 13445–13450 (2008).
Ferdinandusse, S. et al. Subcellular localization and physiological role of α-methylacyl-CoA racemase. J. Lipid Res. 41, 1890–1896 (2000).
Zhang-Gandhi, C. X. & Drew, P. D. Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J. Neuroimmunol. 183, 50–59 (2007).
Wang, L. et al. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc. Natl Acad. Sci. USA 99, 13878–13883 (2002).
Yvan-Charvet, L. et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 117, 3900–3908 (2007).
Pericak-Vance, M. A. et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am. J. Hum. Genet. 48, 1034–1050 (1991).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 (1993).
Rhinn, H. et al. Integrative genomics identifies APOE ε4 effectors in Alzheimer's disease. Nature 500, 45–50 (2013).
Jun, G. et al. Comprehensive search for Alzheimer disease susceptibility loci in the APOE region. Arch. Neurol. 69, 1270–1279 (2012).
Fukumoto, H., Deng, A., Irizarry, M. C., Fitzgerald, M. L. & Rebeck, G. W. Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Aβ levels. J. Biol. Chem. 277, 48508–48513 (2002).
Burns, M. P. et al. The effects of ABCA1 on cholesterol efflux and Aβ levels in vitro and in vivo. J. Neurochem. 98, 792–800 (2006).
Abildayeva, K. et al. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 281, 12799–12808 (2006).
Sun, Y., Yao, J., Kim, T. W. & Tall, A. R. Expression of liver X receptor target genes decreases cellular amyloid β peptide secretion. J. Biol. Chem. 278, 27688–27694 (2003).
Fitz, N. F. et al. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J. Neurosci. 30, 6862–6872 (2010).
Xu, H. E. et al. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc. Natl Acad. Sci. USA 98, 13919–13924 (2001).
Koldamova, R. P. et al. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid β secretion. J. Biol. Chem. 278, 13244–13256 (2003).
Lupton, M. K. et al. The role of ABCA1 gene sequence variants on risk of Alzheimer's disease. J. Alzheimers Dis. 38, 897–906 (2014).
Sun, Y., Wang, N. & Tall, A. R. Regulation of adrenal scavenger receptor-BI expression by ACTH and cellular cholesterol pools. J. Lipid Res. 40, 1799–1805 (1999).
Wang, Y. et al. The selective Alzheimer's disease indicator-1 gene (Seladin-1/DHCR24) is a liver X receptor target gene. Mol. Pharmacol. 74, 1716–1721 (2008).
Greeve, I. et al. The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer's disease-associated neurodegeneration and oxidative stress. J. Neurosci. 20, 7345–7352 (2000).
Waterham, H. R. et al. Mutations in the 3β-hydroxysterol Δ24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 69, 685–694 (2001).
Schultz, J. R. et al. Role of LXRs in control of lipogenesis. Genes Dev. 14, 2831–2838 (2000).
Terasaka, N. et al. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 536, 6–11 (2003).
Hu, X. et al. LXRβ activation increases intestinal cholesterol absorption, leading to an atherogenic lipoprotein profile. J. Intern. Med. 272, 452–464 (2012).
Williams, S. et al. X-ray crystal structure of the liver X receptor β ligand binding domain: regulation by a histidine-tryptophan switch. J. Biol. Chem. 278, 27138–27143 (2003).
Janowski, B. A. et al. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl Acad. Sci. USA 96, 266–271 (1999).
Ratni, H. et al. Discovery of tetrahydro-cyclopenta[b]indole as selective LXRs modulator. Bioorg. Med. Chem. Lett. 19, 1654–1657 (2009).
Hu, B. et al. Identification of phenylsulfone-substituted quinoxaline (WYE-672) as a tissue selective liver X-receptor (LXR) agonist. J. Med. Chem. 53, 3296–3304 (2010).
Griffett, K., Solt, L. A., El- Gendy Bel, D., Kamenecka, T. M. & Burris, T. P. A liver-selective LXR inverse agonist that suppresses hepatic steatosis. ACS Chem. Biol. 8, 559–567 (2013).
Tall, A. R. Plasma cholesteryl ester transfer protein. J. Lipid Res. 34, 1255–1274 (1993).
Nishina, P. M., Verstuyft, J. & Paigen, B. Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J. Lipid Res. 31, 859–869 (1990).
Paigen, B., Ishida, B. Y., Verstuyft, J., Winters, R. B. & Albee, D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis 10, 316–323 (1990).
Laffitte, B. A. et al. Autoregulation of the human liver X receptor α promoter. Mol. Cell. Biol. 21, 7558–7568 (2001).
Riddell, D. R. et al. The LXR agonist TO901317 selectively lowers hippocampal Aβ42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol. Cell Neurosci. 34, 621–628 (2007).
Cramer, P. E. et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506 (2012).
Zhang, C. & Duvic, M. Treatment of cutaneous T-cell lymphoma with retinoids. Dermatol. Ther. 19, 264–271 (2006).
Katz, A. et al. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J. Clin. Pharmacol. 49, 643–649 (2009). This is the first published study of the effects of an LXR agonist in humans.
Quinet, E. M. et al. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J. Lipid Res. 50, 2358–2370 (2009).
Shih, D. M. et al. Hyodeoxycholic acid improves HDL function and inhibits atherosclerotic lesion formation in LDLR-knockout mice. FASEB J. 27, 3805–3817 (2013).
Acknowledgements
P.T. is an investigator of the Howard Hughes Medical Institute and his research was supported by the US National Institutes of Health (NIH) grant HL-066088. The research of C.H. is supported by a grant from the American Heart Association (13BGIA17110079).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Low-density lipoprotein receptor
-
(LDLR). The primary receptor for LDL clearance from the circulation; it is found on the plasma membrane, with highest levels of expression in the liver. Genetic mutations in this protein are linked to familial hypercholesterolaemia.
- Oxysterols
-
Derivatives of cholesterol, some of which act as endogenous ligands for the liver X receptor.
- Retinoid X receptors
-
(RXRs). Members of the nuclear receptor superfamily that exist as three subtypes — RXRα, RXRβ, RXRγ — and bind to the endogenous ligand 9-cis retinoic acid.
- ATP-binding cassette subfamily A member 1
-
(ABCA1). A membrane-bound transporter with high levels of expression in the liver and macrophages. ABCA1 transports cholesterol and phospholipids to apolipoproteins. Mutations in the protein cause Tangier disease.
- DR4 response element
-
A DNA-binding motif that consists of four base pairs separating a repeating 6-mer. The canonical direct repeat 4 (DR4) motif bound by liver X receptors is AGGTCAnnnnAGGTCA (where 'n' refers to any nucleotide).
- Apolipoprotein E
-
(APOE). A protein that is primarily synthesized in the liver and functions to transport lipoproteins. Mutations in APOE have been associated with lipid disorders and Alzheimer's disease.
- Reverse cholesterol transport
-
A pathway in which cholesterol is returned from the periphery back to the liver for redistribution or excretion.
- Phospholipid transfer protein
-
(PLTP). A protein that transfers phospholipids from triglyceride-rich lipoproteins to high-density lipoproteins, and is highly expressed in the liver.
- Cholesteryl ester transfer protein
-
(CETP). A protein that transfers cholesterol esters from high-density lipoprotein (HDL) to triglyceride-rich lipoproteins in humans.
- ob/ob mouse
-
A genetically modified mouse that is leptin-deficient and widely used as a model of obesity and insulin resistance.
- Foam cells
-
Lipid-laden macrophages that are found in atherosclerotic lesions.
- FERM domain
-
A protein–protein interaction domain (containing 4.1 protein, ezrin, radixin and moesin) that is found in membrane-associated proteins.
- RING domain
-
A zinc finger motif that is characteristic of E3 ubiquitin ligases.
- Smith–Lemli–Opitz syndrome
-
A syndrome that is characterized by 7-dehydrocholesterol reductase deficiency. It is autosomal recessive and phenotypically characterized by distinct facial features, small head size and intellectual disabilities.
- Niemann–Pick type C disease
-
A disease that is caused by a genetic mutation in either Niemann–Pick C1 protein (NPC1) or NPC2, resulting in the cellular accumulation of unesterified cholesterol.
- Amyloid-β peptides
-
The primary element of the amyloid plaques that are found in patients with Alzheimer's disease.
- APP-transgenic mice
-
Genetically modified mice that overexpress a form of amyloid precursor protein (APP) with mutations that are associated with familial Alzheimer's disease.
- Full agonists
-
Ligands that bind to and activate a receptor to achieve a maximal response for that receptor.
- Inverse agonists
-
Ligands that bind to a receptor and induce a response that is opposite to that induced by an agonist of that receptor: that is, they reduce the basal activity of the receptor.
Rights and permissions
About this article
Cite this article
Hong, C., Tontonoz, P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov 13, 433–444 (2014). https://doi.org/10.1038/nrd4280
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4280
This article is cited by
-
C1q/Tumor Necrosis Factor-Related Protein-9 Enhances Macrophage Cholesterol Efflux and Improves Reverse Cholesterol Transport via AMPK Activation
Biochemical Genetics (2024)
-
Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
CD11c+ microglia promote white matter repair after ischemic stroke
Cell Death & Disease (2023)
-
Central nervous system macrophages in progressive multiple sclerosis: relationship to neurodegeneration and therapeutics
Journal of Neuroinflammation (2022)
-
Lipid metabolism and storage in neuroglia: role in brain development and neurodegenerative diseases
Cell & Bioscience (2022)