Key Points
-
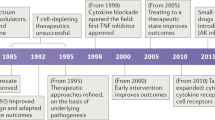
The recent advances in our knowledge of the proteins and the signalling pathways that are involved in innate immunity, together with the identification of cognate ligands for protein receptors of the innate immune system, provide new targets for drug development, which might be useful for combating various human diseases.
-
The key receptors in the innate immune system are the Toll-like receptors (TLRs), of which there are ten in humans, and the nucleotide-binding oligomerization domain (NOD) proteins NOD1 and NOD2, which are intracellular sensors of bacterial products.
-
The leucine-rich repeats present in both TLRs and NOD1/2, and the Toll/interleukin-1 receptor (TIR) domains of TLRs, could be exploited as drug targets.
-
Agonists or antagonists that target the ligand-binding sites of these receptors might also yield new drugs. Small-molecule agonists that target the TLRs have already been developed.
-
The heteromeric nature of the signalling complexes that contain TLRs in association with other molecules might also provide targets for the development of therapeutic drugs.
-
Although further developments could be hampered by limitations on our ability to express proteins for high-throughput screens and by the problems associated with blocking protein–protein interactions, it should be possible to build on the initial success of imidazole quinoline molecules that target TLR7 and TLR8, and the modified CpG-containing compounds that target TLR9.
Abstract
Proteins that recognize the components and products of microorganisms have an important role in innate immunity. Here, I focus on recent advances in our understanding of the function of several such protein families. In particular, I consider how members of the TLR (Toll-like receptor), NOD (nucleotide-binding oligomerization domain)-protein and MyD88 (myeloid differentiation primary-response protein 88) families are providing emerging opportunities for the development of new therapeutics that modify innate immune responses in ways which benefit the host.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Yamamoto, M., Takeda, K. & Akira, S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol. Immunol. 40, 861–868 (2004).
Beutler, B., Hoebe, K., Du, X. & Ulevitch, R. J. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74, 479–485 (2003).
Underhill, D. M. Toll-like receptors: networking for success. Eur. J. Immunol. 33, 1767–1775 (2003).
O'Neill, L. A. The role of MyD88-like adapters in Toll-like receptor signal transduction. Biochem. Soc. Trans. 31, 643–647 (2003).
Dunne, A. & O'Neill, L. A. The interleukin-1 receptor/Toll–like receptor superfamily: signal transduction during inflammation and host defense. Sci. STKE [online] 2003, re3 (2003).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Chamaillard, M., Girardin, S. E., Viala, J. & Philpott, D. J. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell. Microbiol. 5, 581–592 (2003).
Inohara, N. & Nunez, G. NODs: intracellular proteins involved in inflammation and apoptosis. Nature Rev. Immunol. 5, 371–382 (2003).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nature Rev. Immunol. 4, 499–511 (2004).
Girardin, S. E. et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278, 41702–41708 (2003).
Chamaillard, M. et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nature Immunol. 4, 702–707 (2003).
Girardin, S. E. et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300, 1584–1587 (2003). Provides the first definition of the structural requirements of the NOD1 ligand.
O'Neill, L. A. et al. Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors. J. Endotoxin Res. 9, 55–59 (2003).
Martin, M. U. & Wesche, H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Biophys. Acta 1592, 265–268 (2002).
O'Neill, L. A., Fitzgerald, K. A. & Bowie, A. G. The Toll–IL-1 receptor adaptor family grows to five members. Trends Immunol. 24, 286–290 (2003).
Ogura, Y. et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276, 4812–4818 (2001).
Kobayashi, K. et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416, 194–199 (2002). Provides important evidence implicating RICK in both TLR- and NOD-protein signalling pathways.
Hoffmann, J. A. The immune response of Drosophila. Nature 426, 33–38 (2003).
Gobert, V. et al. Dual activation of the Drosophila Toll pathway by two pattern recognition receptors. Science 302, 2126–2130 (2003).
Imler, J. L. & Hoffmann, J. A. Toll signaling: the TIReless quest for specificity. Nature Immunol. 4, 105–106 (2003).
Gottar, M. et al. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416, 640–644 (2002). Provides a new paradigm that describes different arms of the innate immune response in insects.
Zhang, D. et al. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004).
Nishiya, T. & DeFranco, A. L. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J. Biol. Chem. 279, 19008–19017 (2004).
Miyake, K. Endotoxin recognition molecules, Toll-like receptor 4–MD-2. Semin. Immunol. 16, 11–16 (2004).
Akashi, S. et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4–MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198, 1035–1042 (2003).
Wetzler, L. M. The role of Toll-like receptor 2 in microbial disease and immunity. Vaccine 21, S55–S60 (2003).
Kirschning, C. J. & Schumann, R. R. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr. Top. Microbiol. Immunol. 270, 121–144 (2002).
Hajjar, A. M. et al. Cutting edge: Functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166, 15–19 (2001).
Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl Acad. Sci. USA 97, 13766–13771 (2000). Provides key data showing how TLRs comprise heteromeric subunits that determine ligand specificity.
Mizel, S. B., Honko, A. N., Moors, M. A., Smith, P. S. & West, A. P. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J. Immunol. 170, 6217–6223 (2003).
Schulze-Lefert, P. Plant immunity: the origami of receptor activation. Curr. Biol. 14, R22–R24 (2004).
Nimchuk, Z., Eulgem, T., Holt, B. F. & Dangl, J. L. Recognition and response in the plant immune system. Annu. Rev. Genet. 37, 579–609 (2003).
Martin, G. B., Bogdanove, A. J. & Sessa, G. Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 54, 23–61 (2003).
Martinon, F. & Tschopp, J. Inflammatory caspases; linking an intracellular innate immune system to autoinflammatory diseases. Cell 117, 561–574 (2004).
Tanabe, T. et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 23, 1587–1597 (2004).
Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520 (2003).
Krug, A. et al. Herpes simplex virus type 1 activates murine natural interferon producing cells through Toll-like receptor 9. Blood 103, 1433–1437 (2004).
Kaisho, T. & Akira, S. Regulation of dendritic cell function through Toll-like receptors. Curr. Mol. Med. 3, 373–385 (2003).
El Biaze, M. et al. T cell activation, from atopy to asthma: more a paradox than a paradigm. Allergy 58, 844–853 (2003).
Hussain, I. & Kline, J. N. CpG oligodeoxynucleotides: a novel therapeutic approach for atopic disorders. Curr. Drug Targets Inflamm. Allergy 2, 199–205 (2003).
Kuchroo, V. K., Umetsu, D. T., DeKruyff, R. H. & Freeman, G. J. The TIM gene family: emerging roles in immunity and disease. Nature Rev. Immunol. 3, 454–462 (2003).
Melief, C. J. Strategies for immunotherapy of cancer. Adv. Immunol. 75, 235–282 (2000).
Hess, J., Schaible, U., Raupach, B. & Kaufmann, S. H. Exploiting the immune system: toward new vaccines against intracellular bacteria. Adv. Immunol. 75, 1–88 (2000).
Kaisho, T. & Akira, S. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 1589, 1–13 (2002).
Jiang, Z. H. & Koganty, R. R. Synthetic vaccines: the role of adjuvants in immune targeting. Curr. Med. Chem. 10, 1423–1439 (2003).
Evans, J. T. et al. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi. 529. Expert Rev. Vaccines 2, 219–229 (2003).
Persing, D. H. Taking Toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10, S32–S37 (2002).
Stover, A. G. et al. Structure-activity relationship of synthetic Toll-like receptor 4 agonists. J. Biol. Chem. 279, 4440–4449 (2004).
Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004). Identifies a naturally occurring ligand for TLR 7 and TLR 8.
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004). Identifies a naturally occurring ligand for TLR7.
Skinner, R. B. Jr. Imiquimod. Dermatol. Clin. 21, 291–300 (2003).
Hengge, U. R. & Cusini, M. Topical immunomodulators for the treatment of external genital warts, cutaneous warts and molluscum contagiosum. Br. J. Dermatol. 149 (Suppl. 66), 15–19 (2003).
Bernard, H. U. Established and potential strategies against papillomavirus infections. J. Antimicrob. Chemother. 53, 137–139 (2004).
Akira, S. & Hemmi, H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85, 85–95 (2003).
Heil, F. et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 33, 2987–2997 (2003).
Hemmi, H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature Immunol. 3, 196–200 (2002).
Baldridge, J. R. et al. Immunostimulatory activity of aminoalkyl glucosaminide 4-phosphates (AGPs): induction of protective innate immune responses by RC-524 and RC-529. J. Endotoxin Res. 8, 453–458 (2002).
Kurt-Jones, E. A. et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature Immunol. 1, 398–401 (2000). Among the first reports to indicate a role for the innate immune system in antiviral responses.
Doyle, S. et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17, 251–263 (2002).
Hoebe, K. & Beutler, B. LPS, dsRNA and the interferon bridge to adaptive immune responses: Trif, Tram, and other TIR adaptor proteins. J. Endotoxin Res. 10, 130–136 (2004).
Tabeta, K. et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl Acad. Sci. USA 101, 3516–3521 (2004).
Hoebe, K. et al. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nature Immunol. 4, 1223–1229 (2003).
Beutler, B. et al. Lps2 and signal transduction in sepsis: at the intersection of host responses to bacteria and viruses. Scand. J. Infect. Dis. 35, 563–567 (2003).
Honda, K. et al. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl Acad. Sci. USA 100, 10872–10877 (2003).
Matsumoto, M., Funami, K., Oshiumi, H. & Seya, T. Toll-like receptor 3: a link between Toll-like receptor, interferon and viruses. Microbiol. Immunol. 48, 147–154 (2004).
Heit, A. et al. Cutting edge: Toll-like receptor 9 expression is not required for CpG DNA-aided cross-presentation of DNA-conjugated antigens but essential for cross-priming of CD8 T cells. J. Immunol. 170, 2802–2805 (2003).
Heit, A. et al. CpG-DNA aided cross-priming by cross-presenting B cells. J. Immunol. 172, 1501–1507 (2004).
Speigelberg, H. L., Horner, A. A., Takabayashi, K. & Raz, E. Allergen-immunostimulatory oligodeoxynucleotide conjugate: a novel allergoid for immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2, 547–551 (2002).
Karin, M., Yamamoto, Y. & Wang, Q. M. The IKK NF-κB system: a treasure trove for drug development. Nature Rev. Drug Discov. 3, 17–26 (2004).
Arkin, M. R. & Wells, J. A. Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nature Rev. Drug Discov. 3, 301–317 (2004).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Bartfai, T. et al. A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc. Natl Acad. Sci. USA 100, 7971–7976 (2003). Provides the first evidence indicating that it is possible to target TIR–TIR domain interactions with a small molecule.
Heikenwalder, M. et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nature Med. 10, 187–192 (2004).
Hoebe, K. et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424, 743–748 (2003). This seminal paper describes the use of forward genetics to decipher TLR-signalling pathways and identifies Lps2 as a gene encoding TRIF.
Kawai, T., Adachi, O., Ogawa, T., Takeda, K. & Akira, S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122 (1999).
Yamamoto, M. et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nature Immunol. 4, 1144–1150 (2003).
Fitzgerald, K. A. et al. LPS–TLR4 signaling to IRF-3/7 and NF-κB involves the Toll adapters TRAM and TRIF. J. Exp. Med. 198, 1043–1055 (2003).
Drachenberg, K. J., Heinzkill, M., Urban, E. & Woroniecki, S. R. Efficacy and tolerability of short-term specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A (MPL) for children and adolescents. Allergol. Immunopathol. (Madr.) 31, 270–277 (2003).
Mothes, N. et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin. Exp. Allergy 33, 1198–1208 (2003).
Bienzle, U. et al. Immunization with an adjuvant hepatitis B vaccine after liver transplantation for hepatitis B-related disease. Hepatology 38, 811–819 (2003).
Vernacchio, L. et al. Effect of monophosphoryl lipid A (MPL) on T-helper cells when administered as an adjuvant with pneumocococcal-CRM197 conjugate vaccine in healthy toddlers. Vaccine 20, 3658–3667 (2002).
Lynn, M. et al. Extended in vivo pharmacodynamic activity of E5564 in normal volunteers with experimental endotoxemia. J. Pharmacol. Exp. Ther. 308, 175–181 (2004).
Wong, Y. N. et al. Safety, pharmacokinetics, and pharmacodynamics of E5564, a lipid A antagonist, during an ascending single-dose clinical study. J. Clin. Pharmacol. 43, 735–742 (2003).
Liang, E. et al. Pharmacokinetics of E5564, a lipopolysaccharide antagonist, in patients with impaired hepatic function. J. Clin. Pharmacol. 43, 1361–1369 (2003).
Agarwala, S. S., Kirkwood, J. M. & Bryant, J. Phase 1, randomized, double-blind trial of 7-allyl-8-oxoguanosine (loxoribine) in advanced cancer. Cytokines Cell. Mol. Ther. 6, 171–176 (2000).
Sauder, D. N., Smith, M. H., Senta-McMillian, T., Soria, I. & Meng T. C. Randomized, single-blind, placebo-controlled study of topical application of the immune response modulator resiquimod in healthy adults. Antimicrob. Agents Chemother. 47, 3846–3852 (2003).
Jones, T. Resiquimod 3M. Curr. Opin. Investig. Drugs 4, 214–218 (2003).
Imiquimod (Aldara) for actinic keratoses. Med. Lett. Drugs Ther. 46, 42–44 (2004).
Prinz, B. M. et al. Treatment of Bowen's disease with imiquimod 5% cream in transplant recipients. Transplantation 77, 790–791 (2004).
Kreuter, A. et al. Treatment of anal intraepithelial neoplasia in patients with acquired HIV with imiquimod 5% cream. J. Am. Acad. Dermatol. 50, 980–981 (2004).
Huber, A. et al. Topical imiquimod treatment for nodular basal cell carcinomas: an open-label series. Dermatol. Surg. 30, 429–430 (2004).
Herbert, W. C. Imiquimod and the treatment of cutaneous T-cell proliferative diseases: at the threshold. Skinmed. 2, 273–274 (2003).
Leifer, C. A., Verthelyi, D. & Klinman, D. M. Heterogeneity in the human response to immunostimulatory CpG oligodeoxynucleotides. J. Immunother. 26, 313–319 (2003).
Tulic, M. K. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J. Allergy Clin. Immunol. 113, 235–241 (2004).
Bohle, B. AIC. Dynavax. Curr. Opin. Investig. Drugs 4, 603–607 (2003).
Immunostimulatory DNA–Dynavax. AIC, Amb a 1 immunostimulatory conjugate, HBV-ISS, ISS 1018, ISS DNA, ISS DNA–dynavax, ISS1, ISS2. Drugs R D 3, 193–196 (2002).
Paul, S. Technology evaluation: CpG-7909, Coley. Curr. Opin. Mol. Ther. 5, 553–559 (2003).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Acknowledgements
I thank J. C. Mathison for comments and editorial support and P. Rutledge for administrative support. This work was supported by the National Institutes of Health, The Charles Dana Foundation and The Novartis Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Richard J. Ulevitch is a consultant for the Corixa Corporation (Seattle, United States), which has developed monophosphoryl-lipid-A-based adjuvants, the Ribi adjuvant system and the synthetic adjuvant RC-529. His laboratory also has a grant that is part of a National Institutes of Health (United States) Program Project in which Corixa workers are the Principal Investigators.
Rights and permissions
About this article
Cite this article
Ulevitch, R. Therapeutics targeting the innate immune system. Nat Rev Immunol 4, 512–520 (2004). https://doi.org/10.1038/nri1396
Issue Date:
DOI: https://doi.org/10.1038/nri1396
This article is cited by
-
HMGB1 and Toll-like receptors: potential therapeutic targets in autoimmune diseases
Molecular Medicine (2023)
-
A Chemically Modified Curcumin (CMC 2.24) Inhibits Nuclear Factor κB Activation and Inflammatory Bone Loss in Murine Models of LPS-Induced Experimental Periodontitis and Diabetes-Associated Natural Periodontitis
Inflammation (2017)
-
Human Granulocyte Macrophage Colony-Stimulating Factor Enhances Antibiotic Susceptibility of Pseudomonas aeruginosa Persister Cells
Scientific Reports (2015)
-
Cross-protective effect of a combined L5 plus L3 Leishmania major ribosomal protein based vaccine combined with a Th1 adjuvant in murine cutaneous and visceral leishmaniasis
Parasites & Vectors (2014)
-
Modulating immunity as a therapy for bacterial infections
Nature Reviews Microbiology (2012)