Key Points
-
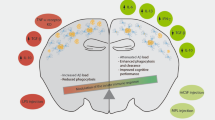
Alzheimer's disease affects more than 20 million people worldwide and is characterized by progressive memory deficits, cognitive impairment and personality changes. It is believed that the main cause of Alzheimer's disease is increased production and accumulation of amyloid-β peptide (amyloid-β1–42).
-
Although inflammation had not been viewed as a hallmark of Alzheimer's disease, amyloid-β deposition activates a potentially pathological innate immune response, including complement, microglial-cell activation and astrocyte proliferation.
-
There is a reduced prevalence of Alzheimer's disease among chronic users of non-steroidal anti-inflammatory drugs (NSAIDs). This might be due to the effects of NSAIDs on the protease that generates amyloid-β1–42 from amyloid precursor protein rather than to anti-inflammatory effects.
-
Immunotherapy in mouse models of Alzheimer's disease by active immunization of amyloid-β or by passive administration of amyloid-β-specific antibodies markedly reduces amyloid-β levels in the brain and reverses behavioural impairment.
-
A human clinical trial of amyloid-β immunization led to meningoencephalitis in some patients with Alzheimer's disease and was discontinued. Nevertheless, in a subset of the clinical trial participants, those with plaque-reactive amyloid-β-specific antibodies in the plasma had a significantly slower rate of cognitive decline than those patients that did not have detectable amyloid-β- specific antibodies.
-
Microglial cells represent a natural mechanism by which protein aggregates and debris can be removed from the brain, and there are multiple routes by which resident microglial cells can be activated to promote clearance of amyloid-β. Evidence from animal models and patients with Alzheimer's disease indicates that microglial-cell activation can lead to amyloid-β clearance.
-
Although there is no clear T-cell response in the brains of patients with Alzheimer's disease, understanding T-cell responses and adaptive immunity has become important in the immunotherapy of Alzheimer's disease. Induction of appropriate T-cell subsets might have a central role in non-antibody-mediated clearance of amyloid-β, most probably by stimulating microglial cells.
Abstract
Although Alzheimer's disease is considered to be a degenerative brain disease, it is clear that the immune system has an important role in the disease process. As discussed in this Review, immune-based therapies that are designed to remove amyloid-β peptide from the brain have produced positive results in animal models of the disease and are being tested in humans with Alzheimer's disease. Although immunotherapy holds great promise for the treatment of Alzheimer's disease, clinical trials of active amyloid-β vaccination of patients with Alzheimer's disease were discontinued after some patients developed meningoencephalitis. New immunotherapies using humoral and cell-based approaches are currently being investigated for the treatment and prevention of Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Braak, H., Braak, E. & Bohl, J. Staging of Alzheimer-related cortical destruction. Eur. Neurol. 33, 403–408 (1993).
Jellinger, K. A. & Bancher, C. AD neuropathology. Neurology 46, 1186–1187 (1996).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Selkoe, D. J. Alzheimer's disease: a central role for amyloid. J. Neuropathol. Exp. Neurol. 53, 438–447 (1994). This article explains the amyloid hypothesis in Alzheimer's disease.
Gouras, G. K., Almeida, C. G. & Takahashi, R. H. Intraneuronal Aβ accumulation and origin of plaques in Alzheimer's disease. Neurobiol. Aging 26, 1235–1244 (2005).
Turner, P. R., O'Connor, K., Tate, W. P. & Abraham, W. C. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 70, 1–32 (2003).
Games, D. et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527 (1995).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996). This article shows a correlation between amyloid-β load and cognitive memory deficits in APP -transgenic mice.
Routtenberg, A. Measuring memory in a mouse model of Alzheimer's disease. Science 277, 839–841 (1997).
Sturchler-Pierrat, C. et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl Acad. Sci. USA 94, 13287–13292 (1997).
Bronfman, F. C., Moechars, D. & Van Leuven, F. Acetylcholinesterase-positive fiber deafferentation and cell shrinkage in the septohippocampal pathway of aged amyloid precursor protein London mutant transgenic mice. Neurobiol. Dis. 7, 152–168 (2000).
Dewachter, I. et al. Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice. Exp. Gerontol. 35, 831–841 (2000).
Kuo, Y. M. et al. Comparative analysis of amyloid-β chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer's disease brains. J. Biol. Chem. 276, 12991–12998 (2001).
Morgan, D. et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408, 982–985 (2000).
Janus, C. et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408, 979–982 (2000).
McGeer, P. L. & McGeer, E. G. Inflammation, autotoxicity and Alzheimer disease. Neurobiol. Aging 22, 799–809 (2001). This article describes the innate immune response in Alzheimer's disease.
Akiyama, H. et al. Inflammation and Alzheimer's disease. Neurobiol. Aging 21, 383–421 (2000).
Togo, T. et al. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J. Neuroimmunol. 124, 83–92 (2002).
McGeer, E. G. & McGeer, P. L. Innate immunity in Alzheimer's disease: a model for local inflammatory reactions. Mol. Interv. 1, 22–29 (2001). References 18 and 19 show that lowering amyloid-β by anti-amyloid therapy improves cognitive behaviour in APP -transgenic mice.
Rogers, J. et al. Complement activation by β-amyloid in Alzheimer disease. Proc. Natl Acad. Sci. USA 89, 10016–10020 (1992).
Webster, S. & Rogers, J. Relative efficacies of amyloid β peptide (Aβ) binding proteins in Aβ aggregation. J. Neurosci. Res. 46, 58–66 (1996).
McGreal, E. & Gasque, P. Structure-function studies of the receptors for complement C1q. Biochem. Soc. Trans. 30, 1010–1014 (2002).
Hafer-Macko, C. E., Dyck, P. J. & Koski, C. L. Complement activation in acquired and hereditary amyloid neuropathy. J. Peripher. Nerv. Syst. 5, 131–139 (2000).
Webster, S. D. et al. Antibody-mediated phagocytosis of the amyloid β-peptide in microglia is differentially modulated by C1q. J. Immunol. 166, 7496–7503 (2001).
El Khoury, J. et al. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature 382, 716–719 (1996). A description of the importance of scavenger receptors on microglial cells for amyloid uptake.
Minghetti, L. Role of inflammation in neurodegenerative diseases. Curr. Opin. Neurol. 18, 315–321 (2005).
Bianca, V. D., Dusi, S., Bianchini, E., Dal Pra, I. & Rossi, F. β-amyloid activates the O2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer's disease. J. Biol. Chem. 274, 15493–15499 (1999).
Weldon, D. T. et al. Fibrillar β-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J. Neurosci. 18, 2161–2173 (1998).
McGeer, E. G. & McGeer, P. L. The importance of inflammatory mechanisms in Alzheimer disease. Exp. Gerontol. 33, 371–378 (1998).
Van Muiswinkel, F. L. et al. The amino-terminus of the amyloid-β protein is critical for the cellular binding and consequent activation of the respiratory burst of human macrophages. J. Neuroimmunol. 96, 121–130 (1999).
Ishii, K. et al. Subacute NO generation induced by Alzheimer's β-amyloid in the living brain: reversal by inhibition of the inducible NO synthase. FASEB J. 14, 1485–1489 (2000).
Heneka, M. T. et al. Induction of nitric oxide synthase and nitric oxide-mediated apoptosis in neuronal PC12 cells after stimulation with tumour necrosis factor-α/lipopolysaccharide. J. Neurochem. 71, 88–94 (1998).
Combs, C. K., Karlo, J. C., Kao, S. C. & Landreth, G. E. β-Amyloid stimulation of microglia and monocytes results in TNFα-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 21, 1179–1188 (2001).
Butovsky, O., Talpalar, A. E., Ben-Yaakov, K. & Schwartz, M. Activation of microglia by aggregated β-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-γ and IL-4 render them protective. Mol. Cell. Neurosci. 29, 381–393 (2005).
Streit, W. J. Microglia and Alzheimer's disease pathogenesis. J. Neurosci. Res. 77, 1–8 (2004).
Nicoll, J. A. et al. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nature Med. 9, 448–452 (2003). A case report of neuropathology in a patient who developed meningoencephalitis following immunization with amyloid-β peptide.
Akiyama, H. & McGeer, P. L. Specificity of mechanisms for plaque removal after Aβ immunotherapy for Alzheimer disease. Nature Med. 10, 117–119 (2004).
Weiner, H. L. & Selkoe, D. J. Inflammation and therapeutic vaccination in CNS diseases. Nature 420, 879–884 (2002).
Monsonego, A. & Weiner, H. L. Immunotherapeutic approaches to Alzheimer's disease. Science 302, 834–838 (2003).
Meda, L., Baron, P. & Scarlato, G. Glial activation in Alzheimer's disease: the role of Aβ and its associated proteins. Neurobiol. Aging 22, 885–893 (2001).
Bamberger, M. E. & Landreth, G. E. Microglial interaction with β-amyloid: implications for the pathogenesis of Alzheimer's disease. Microsc. Res. Tech. 54, 59–70 (2001).
Kitazawa, M., Yamasaki, T. R. & Laferla, F. M. Microglia as a potential bridge between the amyloid β-peptide and Tau. Ann. N.Y. Acad. Sci. 1035, 85–103 (2004).
DeWitt, D. A., Perry, G., Cohen, M., Doller, C. & Silver, J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease. Exp. Neurol. 149, 329–340 (1998).
Wyss-Coray, T. et al. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nature Med. 9, 453–457 (2003).
Koistinaho, M. et al. Apolipoprotein E promotes astrocyte co-localization and degradation of deposited amyloid-β peptides. Nature Med. 10, 719–726 (2004).
Dolev, I. & Michaelson, D. M. A nontransgenic mouse model shows inducible amyloid-β (Aβ) peptide deposition and elucidates the role of apolipoprotein E in the amyloid cascade. Proc. Natl Acad. Sci. USA 101, 13909–13914 (2004).
McGeer, P. L., Schulzer, M. & McGeer, E. G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology 47, 425–432 (1996).
Myriad. Molecule of the month. MPC-7869 (Flurizan). Drug News Perspect. 18, 141 (2005).
Yasojima, K., Schwab, C., McGeer, E. G. & McGeer, P. L. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 830, 226–236 (1999).
Xiang, Z. et al. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer's disease neuropathology. Gene Expr. 10, 271–278 (2002).
Nogawa, S., Zhang, F., Ross, M. E. & Iadecola, C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 17, 2746–2755 (1997).
Hoozemans, J. J., Veerhuis, R., Rozemuller, A. J. & Eikelenboom, P. Non-steroidal anti-inflammatory drugs and cyclooxygenase in Alzheimer's disease. Curr. Drug Targets 4, 461–468 (2003).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 (2001). A description of lowering of amyloid load in APP -transgenic mice by NSAIDs that is independent of their anti-inflammatory activity.
Takahashi, Y. et al. Sulindac sulfide is a noncompetitive γ-secretase inhibitor that preferentially reduces Aβ42 generation. J. Biol. Chem. 278, 18664–18670 (2003).
Reid, G. et al. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl Acad. Sci. USA 100, 9244–9249 (2003).
Warner, T. D. & Mitchell, J. A. Nonsteroidal antiinflammatory drugs inhibiting prostanoid efflux: as easy as ABC? Proc. Natl Acad. Sci. USA 100, 9108–9110 (2003).
Xu, H., Sweeney, D., Greengard, P. & Gandy, S. Metabolism of Alzheimer β-amyloid precursor protein: regulation by protein kinase A in intact cells and in a cell-free system. Proc. Natl Acad. Sci. USA 93, 4081–4084 (1996).
Marambaud, P., Ancolio, K., Lopez-Perez, E. & Checler, F. Proteasome inhibitors prevent the degradation of familial Alzheimer's disease-linked presenilin 1 and potentiate Aβ42 recovery from human cells. Mol. Med. 4, 147–157 (1998).
Aisen, P. S. et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289, 2819–2826 (2003).
Jantzen, P. T. et al. Microglial activation and β-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 22, 2246–2254 (2002).
Solomon, B., Koppel, R., Hanan, E. & Katzav, T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer β-amyloid peptide. Proc. Natl Acad. Sci. USA 93, 452–455 (1996).
Solomon, B., Koppel, R., Frenkel, D. & Hanan-Aharon, E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc. Natl Acad. Sci. USA 94, 4109–4112 (1997). A demonstration that antibody can reduce amyloid-β fibril formation in vitro.
Frenkel, D., Balass, M. & Solomon, B. N-terminal EFRH sequence of Alzheimer's β-amyloid peptide represents the epitope of its anti-aggregating antibodies. J. Neuroimmunol. 88, 85–90 (1998).
Frenkel, D., Balass, M., Katchalski-Katzir, E. & Solomon, B. High affinity binding of monoclonal antibodies to the sequential epitope EFRH of β-amyloid peptide is essential for modulation of fibrillar aggregation. J. Neuroimmunol. 95, 136–142 (1999).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999). A demonstration that immunization of APP -transgenic mice with amyloid-β clears amyloid.
Weiner, H. L. et al. Nasal administration of amyloid-β peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann. Neurol. 48, 567–579 (2000). Mucosal amyloid immunization reduces amyloid plaques in vivo.
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Med. 6, 916–919 (2000). Passive administration of amyloid-β-specific antibody can clear amyloid plaques in vivo.
Dodart, J. C. et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer's disease model. Nature Neurosci. 5, 452–457 (2002).
DeMattos, R. B. et al. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 98, 8850–8855 (2001).
Sigurdsson, E. M., Scholtzova, H., Mehta, P. D., Frangione, B. & Wisniewski, T. Immunization with a nontoxic/nonfibrillar amyloid-β homologous peptide reduces Alzheimer's disease-associated pathology in transgenic mice. Am. J. Pathol. 159, 439–447 (2001).
Vehmas, A. K. et al. β-amyloid peptide vaccination results in marked changes in serum and brain Aβ levels in APPswe/PS1ΔE9 mice, as detected by SELDI-TOF-based ProteinChip technology. DNA Cell Biol. 20, 713–721 (2001).
Li, Q. et al. Overcoming antigen masking of anti-amyloid-β antibodies reveals breaking of B cell tolerance by virus-like particles in amyloid-β immunized amyloid precursor protein transgenic mice. BMC Neurosci. 5, 21 (2004).
Das, P. et al. Amyloid-β immunization effectively reduces amyloid deposition in FcRγ−/− knock-out mice. J. Neurosci. 23, 8532–8538 (2003).
Zhang, J. et al. A novel recombinant adeno-associated virus vaccine reduces behavioural impairment and β-amyloid plaques in a mouse model of Alzheimer's disease. Neurobiol. Dis. 14, 365–379 (2003).
Lemere C. A. et al. Evidence for peripheral clearance of cerebral Aβ protein following chronic, active Aβ immunization in PSAPP mice. Neurobiol. Dis. 14, 10–18 (2003).
Schiltz, J. G. et al. Antibodies from a DNA peptide vaccination decrease the brain amyloid burden in a mouse model of Alzheimer's disease. J. Mol. Med. 82, 706–714 (2004).
Hara, H. et al. Development of a safe oral Aβ vaccine using recombinant adeno-associated virus vector for Alzheimer's disease. J. Alzheimers Dis. 6, 483–488 (2004).
Frenkel, D., Katz, O. & Solomon, B. Immunization against Alzheimer's β-amyloid plaques via EFRH phage administration. Proc. Natl Acad. Sci. USA 97, 11455–11459 (2000).
Frenkel, D., Dewachter, I., Van Leuven, F. & Solomon, B. Reduction of β-amyloid plaques in brain of transgenic mouse model of Alzheimer's disease by EFRH-phage immunization. Vaccine 21, 1060–1065 (2003).
Lavie, V. et al. EFRH-phage immunization of Alzheimer's disease animal model improves behavioural performance in Morris water maze trials. J. Mol. Neurosci. 24, 105–113 (2004).
Lee, E. B. et al. Targeting Aβ oligomers by passive immunization with a conformation selective monoclonal antibody improves learning and memory in APP transgenic mice. J. Biol. Chem. 281, 4292–4299 (2005).
Levites, Y. et al. Anti-Aβ42- and anti-Aβ40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J. Clin. Invest. 116, 193–201 (2006).
McLaurin, J. et al. Therapeutically effective antibodies against amyloid-β peptide target amyloid-β residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nature Med. 8, 1263–1269 (2002).
Oddo, S., Billings, L., Kesslak, J. P., Cribbs, D. H. & LaFerla, F. M. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43, 321–332 (2004).
Morgan, D. & Gitter, B. D. Evidence supporting a role for anti-Aβ antibodies in the treatment of Alzheimer's disease. Neurobiol. Aging 25, 605–608 (2004).
Klyubin, I. et al. Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nature Med. 11, 556–561 (2005).
Wilcock, D. M. et al. Microglial activation facilitates Aβ plaque removal following intracranial anti-Aβ antibody administration. Neurobiol. Dis. 15, 11–20 (2004).
Gilman, S. et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64, 1553–1562 (2005). Clinical and pathological effects following amyloid-β immunization of humans are described.
Bayer, A. J. et al. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology 64, 94–101 (2005).
Orgogozo, J. M. et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 61, 46–54 (2003).
Hock, C. et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer's disease. Neuron 38, 547–554 (2003). Cognitive improvement in patients with Alzheimer's disease with high amyloid-β-specific antibody titres following amyloid-β immunization.
Fox, N. C. et al. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64, 1563–1572 (2005).
Ferrer, I., Boada Rovira, M., Sanchez Guerra, M. L., Rey, M. J. & Costa-Jussa, F. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer's disease. Brain Pathol. 14, 11–20 (2004).
Masliah, E. et al. Aβ vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology 64, 129–131 (2005).
Monsonego, A. et al. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 112, 415–422 (2003). Elderly subjects and Alzheimer's disease patients have increased T-cell responses to amyloid-β in peripheral blood.
O'Toole, M. et al. Risk factors associated with β-amyloid1–42 immunotherapy in preimmunization gene expression patterns of blood cells. Arch. Neurol. 62, 1531–1536 (2005).
Schenk, D., Hagen, M. & Seubert, P. Current progress in β-amyloid immunotherapy. Curr. Opin. Immunol. 16, 599–606 (2004).
Pfeifer, M. et al. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science 298, 1379 (2002).
DiCarlo, G., Wilcock, D., Henderson, D., Gordon, M. & Morgan, D. Intrahippocampal LPS injections reduce Aβ load in APP+PS1 transgenic mice. Neurobiol. Aging 22, 1007–1012 (2001).
Wyss-Coray, T. et al. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc. Natl Acad. Sci. USA 99, 10837–10842 (2002).
Wilcock, D. M. et al. Intracranially administered anti-Aβ antibodies reduce β-amyloid deposition by mechanisms both independent of and associated with microglial activation. J. Neurosci. 23, 3745–37451 (2003).
Frenkel, D., Maron, R., Burt, D. S. & Weiner, H. L. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears β-amyloid in a mouse model of Alzheimer disease. J. Clin. Invest. 115, 2423–2433 (2005). Antibody-independent reduction of amyloid in APP -transgenic mice following microglial-cell activation by myelin antigens and glatiramer acetate.
Nakagawa, Y. et al. Brain trauma in aged transgenic mice induces regression of established Aβ deposits. Exp. Neurol. 163, 244–252 (2000).
Wyss-Coray, T. et al. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nature Med. 7, 612–618 (2001).
Rogers, J., Strohmeyer, R., Kovelowski, C. J. & Li, R. Microglia and inflammatory mechanisms in the clearance of amyloid β peptide. Glia 40, 260–269 (2002).
Bacskai, B. J. et al. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nature Med. 7, 369–372 (2001).
Mitrasinovic, O. M. & Murphy, G. M. Jr. Microglial overexpression of the M-CSF receptor augments phagocytosis of opsonized Aβ. Neurobiol. Aging 24, 807–815 (2003).
Bacskai, B. J. et al. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. J. Neurosci. 22, 7873–7878 (2002).
Frenkel, D., Solomon, B. & Benhar, I. Modulation of Alzheimer's β-amyloid neurotoxicity by site-directed single-chain antibody. J. Neuroimmunol. 106, 23–31 (2000).
Thomas, C. J. et al. Evidence of a trimolecular complex involving LPS, LPS binding protein and soluble CD14 as an effector of LPS response. FEBS Lett. 531, 184–188 (2002).
Hauss-Wegrzyniak, B., Vraniak, P. D. & Wenk, G. L. LPS-induced neuroinflammatory effects do not recover with time. Neuroreport 11, 1759–1763 (2000).
Stalder, A. K. et al. Lipopolysaccharide-induced IL-12 expression in the central nervous system and cultured astrocytes and microglia. J. Immunol. 159, 1344–1351 (1997).
Sly, L. M. et al. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res. Bull. 56, 581–588 (2001).
Qiao, X., Cummins, D. J. & Paul, S. M. Neuroinflammation-induced acceleration of amyloid deposition in the APPV717F transgenic mouse. Eur. J. Neurosci. 14, 474–482 (2001).
Sheng, J. G. et al. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid β peptide in APPswe transgenic mice. Neurobiol. Dis. 14, 133–145 (2003).
Herber, D. L. et al. Time-dependent reduction in Aβ levels after intracranial LPS administration in APP transgenic mice. Exp. Neurol. 190, 245–253 (2004).
Quinn, J. et al. Inflammation and cerebral amyloidosis are disconnected in an animal model of Alzheimer's disease. J. Neuroimmunol. 137, 32–41 (2003).
Jones, T. et al. Protollin: a novel adjuvant for intranasal vaccines. Vaccine 22, 3691–3697 (2004).
Coraci, I. S. et al. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to β-amyloid fibrils. Am. J. Pathol. 160, 101–112 (2002).
Bamberger, M. E., Harris, M. E., McDonald, D. R., Husemann, J. & Landreth, G. E. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. J. Neurosci. 23, 2665–2674 (2003).
Porter, J. C. & Hogg, N. Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 8, 390–396 (1998).
Paresce, D. M., Ghosh, R. N. & Maxfield, F. R. Microglial cells internalize aggregates of the Alzheimer's disease amyloid β-protein via a scavenger receptor. Neuron 17, 553–565 (1996).
Iribarren, P. et al. CpG-containing oligodeoxynucleotide promotes microglial cell uptake of amyloid β1–42 peptide by upregulating the expression of the G-protein-coupled receptor mFPR2. FASEB J. 19, 2032–2034 (2005).
Iribarren, P., Zhou, Y., Hu, J., Le, Y. & Wang, J. M. Role of formyl peptide receptor-like 1 (FPRL1/FPR2) in mononuclear phagocyte responses in Alzheimer disease. Immunol. Res. 31, 165–176 (2005).
Verdier, Y., Zarandi, M. & Penke, B. Amyloid β-peptide interactions with neuronal and glial cell plasma membrane: binding sites and implications for Alzheimer's disease. J. Pept. Sci. 10, 229–248 (2004).
Giulian, D. et al. The HHQK domain of β-amyloid provides a structural basis for the immunopathology of Alzheimer's disease. J. Biol. Chem. 273, 29719–29726 (1998).
Xie, L. et al. Alzheimer's β-amyloid peptides compete for insulin binding to the insulin receptor. J. Neurosci. 22, RC221 (2002).
Boland, K., Behrens, M., Choi, D., Manias, K. & Perlmutter, D. H. The serpin-enzyme complex receptor recognizes soluble, nontoxic amyloid-β peptide but not aggregated, cytotoxic amyloid-β peptide. J. Biol. Chem. 271, 18032–18044 (1996).
Qiu, W. Q. et al. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J. Biol. Chem. 273, 32730–32738 (1998).
Yamada, T. et al. Selective localization of gelatinase A, an enzyme degrading β-amyloid protein, in white matter microglia and in Schwann cells. Acta Neuropathol. (Berl) 89, 199–203 (1995).
Simard, A. R., Soulet, D., Gowing, G., Julien, J. P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49, 489–502 (2006).
Schwartz, M., Butovsky, O., Bruck, W. & Hanisch, U. K. Microglial phenotype: is the commitment reversible? Trends Neurosci. 29, 68–74 (2006). A description of the role of different microglial-cell subsets in the CNS.
Monsonego, A., Maron, R., Zota, V., Selkoe, D. J. & Weiner, H. L. Immune hyporesponsiveness to amyloid β-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer's disease. Proc. Natl Acad. Sci. USA 98, 10273–10278 (2001).
Furlan, R. et al. Vaccination with amyloid-β peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain 126, 285–291 (2003).
Monsonego, A. et al. Aβ-induced meningoencephalitis is IFN-γ dependent and is associated with T-cell dependent clearance of Aβ in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 103, 5048–5053 (2006). Description of meningoencephalitis in APP -transgenic mice and reduction of amyloid-β levels mediated by amyloid-β-specific T cells.
Weiner, H. L. et al. Nasal administration of amyloid-β peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann. Neurol. 48, 567–579 (2000).
Tan, J. et al. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nature Neurosci. 5, 1288–1293 (2002).
LaFerla, F. M. & Oddo, S. Alzheimer's disease: Aβ, tau and synaptic dysfunction. Trends Mol. Med. 11, 170–176 (2005).
Yamamoto, M. et al. Overexpression of monocyte chemotactic protein-1/CCL2 in β-amyloid precursor protein transgenic mice show accelerated diffuse β-amyloid deposition. Am. J. Pathol. 166, 1475–1485 (2005).
Fonseca, M. I., Zhou, J., Botto, M. & Tenner, A. J. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer's disease. J. Neurosci. 24, 6457–6465 (2004).
Acknowledgements
We are grateful to D. Selkoe and to J. El Khoury for discussions and critical review of the manuscript. This work is supported by grants from the National Institute of Ageing, National Institutes of Health (to H.L.W.), Alzheimers' Association (to H.L.W and D.F) and a Human Frontier Science Program Fellowship (to D.F.).
In Table 2, the references were missing for the fifth and ninth rows; the fifth row reference should be 139 and the ninth row reference should be 102. This error has since been corrected in the HTML and PDF versions of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Glossary
- Neurofibrillary tangles
-
Neurofibrillary tangles are pathological protein aggregates found in neurons of patients with Alzheimer's disease. Tangles are formed by hyperphosphorylation of a microtubule-associated protein known as Tau, causing it to aggregate in an insoluble form.
- Association cortices
-
The neocortical regions that are not involved in primary sensory or motor processing. They include frontal areas subserving executive functions and temporoparietal areas supporting visuospatial processing.
- Amyloid
-
A general term for a variety of protein aggregates that accumulate as extracellular fibrils of 7–10 nm. They have common structural features, including a β-pleated sheet conformation and the ability to bind dyes such as Congo Red.
- Astrocyte
-
A star-shaped glial cell of the central nervous system that forms a structural and functional interface between non-nervous tissues and neurons. Once activated, astrocytes express glial fibrillary acidic protein at the cell surface.
- Microglial cell
-
A macrophage-lineage cell that is derived from bone marrow and is present in the central nervous system.
- Tau
-
A neuronal protein that binds to microtubules, promoting their assembly and stability.
- Amyloid precursor protein
-
A membrane glycoprotein component of fast axonal transport, from which amyloid-β is cleaved by proteolytic processing.
- Limbic system
-
A collection of cortical and subcortical structures important for processing memory and emotional information. Prominent structures include the hippocampus and amygdala.
- Amyloid fibrils
-
Structures formed by many disease-causing proteins when they aggregate. Amyloid fibrils share common biochemical characteristics such as detergent insolubility, high β-pleated sheet content and a cross β-structure, protease resistance and the ability to bind lipophilic dyes, such as Congo Red, Thioflavin S and Thioflavin T.
- Cerebral amyloid angiopathy
-
A condition in which there is a deposition of amyloid-β1–40 in the walls of the arteries that supply the brain.
- Amyloid plaques
-
Sites of amyloid-β accumulation and dystrophic neurites in the brains of mouse models and patients with Alzheimer's disease.
- Dystrophic neurites
-
Abnormal swelling that develops secondary to neuronal-cell stress in Alzheimer's disease and other neurodegenerative diseases.
- Ramified process
-
Filamentous branches from the cell body that occur in association with activation of astrocytes and microglial cells.
- Soma
-
The largest part of a cell, the cell body of a microglial cell or astrocyte.
- Astrogliosis
-
An increase in the number of astrocytes owing to proliferation at sites of damage in the central nervous system.
- Gliosis
-
The excess growth of neuroglial cells in the brain that usually follows neuronal-cell death and might lead to the formation of scar tissue.
- Fluid percussion injury
-
Increase of fluid in the brain cranial cavity, leading to brain injury.
Rights and permissions
About this article
Cite this article
Weiner, H., Frenkel, D. Immunology and immunotherapy of Alzheimer's disease. Nat Rev Immunol 6, 404–416 (2006). https://doi.org/10.1038/nri1843
Issue Date:
DOI: https://doi.org/10.1038/nri1843
This article is cited by
-
The neuroimmune axis of Alzheimer’s disease
Genome Medicine (2023)
-
Expression analysis of inhibitory B7 family members in Alzheimer’s disease
Metabolic Brain Disease (2023)
-
Low-dose IL-2 Treatment Rescues Cognitive Deficits by Repairing the Imbalance Between Treg and Th17 Cells at the Middle Alzheimer’s Disease Stage
Journal of Neuroimmune Pharmacology (2023)
-
Immunogenicity of MultiTEP platform technology-based Tau vaccine in non-human primates
npj Vaccines (2022)
-
Inflammasome Signaling in the Aging Brain and Age-Related Neurodegenerative Diseases
Molecular Neurobiology (2022)