Abstract
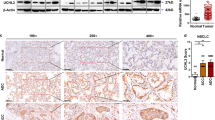
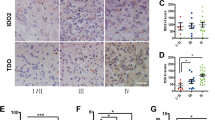
The dioxin/aryl hydrocarbon receptor (AhR) is a transcription factor, which has been attributed a role in human cancerogenesis, cell cycle progression and transforming growth factor-β (TGF-β) signaling. As TGF-β is an important mediator of the malignant phenotype of human gliomas, we studied AhR expression and function in glioma cells. AhR was not only expressed in glioma cells in vitro, but was also detected in human gliomas in vivo by immunohistochemistry, with a predominantly nuclear staining in glioblastomas. The AhR agonist, 3-methylcholanthrene, induced AhR nuclear translocation and upregulated mRNA levels of the AhR target gene, cytochrome P450 1A1 (CYP1A1). Conversely, pharmacological inhibition of AhR using the novel AhR antagonist, CH-223191, or AhR gene silencing using small interfering RNA showed that constitutive AhR activity positively controls TGF-β1, TGF-β2 and latent TGF-β-binding protein-1 protein levels in malignant glioma cells. Moreover, antagonism of AhR reduced clonogenic survival and invasiveness of glioma cells. In contrast, AhR regulates TGF-β signaling negatively in non-neoplastic astrocytes. Thus, the pathogenesis of glioma formation may involve altered AhR regulation of the TGF-β/Smad pathway, and AhR may represent a promising target for the treatment of human malignant gliomas and other diseases associated with pathological TGF-β activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- AhR:
-
aryl hydrocarbon receptor
- ARNT:
-
AhR nuclear translocator
- BrdU:
-
5-bromo-2-deoxyuridine
- CA-AhR:
-
constitutive active AhR
- CI:
-
confidence interval
- CYP1A1:
-
cytochrome P450 1A1
- DRE:
-
dioxin responsive element
- LTBP-1:
-
latent TGF-β-binding protein
- PAS:
-
Per-Arnt-Sim
- pSmad2:
-
phosphorylated Smad2
- TCDD:
-
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TGF-β:
-
transforming growth factor-β
- 3-MC:
-
3-methylcholanthrene
References
Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S et al. (2002). A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci USA 99: 9990–9995.
Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A et al. (2007). High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 11: 147–160.
Carraro G, Albertin G, Forneris M, Nussdorfer GG . (2005). Similar sequence-free amplification of human glyceraldehyde-3-phosphate dehydrogenase for real time RT-PCR applications. Mol Cell Probes 19: 181–186.
Chang X, Fan Y, Karyala S, Schwemberger S, Tomlinson CR, Sartor MA et al. (2007). Ligand-independent regulation of transforming growth factor beta1 expression and cell cycle progression by the aryl hydrocarbon receptor. Mol Cell Biol 27: 6127–6139.
Corchero J, Martin-Partido G, Dallas SL, Fernandez-Salguero PM . (2004). Liver portal fibrosis in dioxin receptor-null mice that overexpress the latent transforming growth factor-beta-binding protein-1. Int J Exp Pathol 85: 295–302.
Denison MS, Nagy SR . (2003). Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43: 309–334.
Elferink CJ . (2003). Aryl hydrocarbon receptor-mediated cell cycle control. Prog Cell Cycle Res 5: 261–267.
Friese MA, Wischhusen J, Wick W, Weiler M, Eisele G, Steinle A et al. (2004). RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res 64: 7596–7603.
Fujisawa-Sehara A, Sogawa K, Yamane M, Fujii-Kuriyama Y . (1987). Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res 15: 4179–4191.
Gomez-Duran A, Mulero-Navarro S, Chang X, Fernandez-Salguero PM . (2006). LTBP-1 blockade in dioxin receptor-null mouse embryo fibroblasts decreases TGF-beta activity: role of extracellular proteases plasmin and elastase. J Cell Biochem 97: 380–392.
Gu YZ, Hogenesch JB, Bradfield CA . (2000). The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 40: 519–561.
Haarmann-Stemmann T, Bothe H, Abel J . (2008). Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem Pharmacol 77: 508–520.
Harper PA, Riddick DS, Okey AB . (2006). Regulating the regulator: factors that control levels and activity of the aryl hydrocarbon receptor. Biochem Pharmacol 72: 267–279.
Huang X, Powell-Coffman JA, Jin Y . (2004). The AHR-1 aryl hydrocarbon receptor and its co-factor the AHA-1 aryl hydrocarbon receptor nuclear translocator specify GABAergic neuron cell fate in C elegans. Development 131: 819–828.
Ito T, Tsukumo S, Suzuki N, Motohashi H, Yamamoto M, Fujii-Kuriyama Y et al. (2004). A constitutively active arylhydrocarbon receptor induces growth inhibition of jurkat T cells through changes in the expression of genes related to apoptosis and cell cycle arrest. J Biol Chem 279: 25204–25210.
Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS et al. (2006). Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol 69: 1871–1878.
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S et al. (1998). Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4: 844–847.
Kress S, Reichert J, Schwarz M . (1998). Functional analysis of the human cytochrome P4501A1 (CYP1A1) gene enhancer. Eur J Biochem 258: 803–812.
Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E et al. (2000). Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA 97: 10442–10447.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al. (2007). The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97–109.
Maier MS, Legare ME, Hanneman WH . (2007). The aryl hydrocarbon receptor agonist 3,3′,4,4′,5-pentachlorobiphenyl induces distinct patterns of gene expression between hepatoma and glioma cells: chromatin remodeling as a mechanism for selective effects. Neurotoxicology 28: 594–612.
Marlowe JL, Puga A . (2005). Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J Cell Biochem 96: 1174–1184.
Mason GG, Witte AM, Whitelaw ML, Antonsson C, McGuire J, Wilhelmsson A et al. (1994). Purification of the DNA binding form of dioxin receptor. Role of the Arnt cofactor in regulation of dioxin receptor function. J Biol Chem 269: 4438–4449.
McGuire J, Okamoto K, Whitelaw ML, Tanaka H, Poellinger L . (2001). Definition of a dioxin receptor mutant that is a constitutive activator of transcription: delineation of overlapping repression and ligand binding functions within the PAS domain. J Biol Chem 276: 41841–41849.
Mimura J, Fujii-Kuriyama Y . (2003). Xenobiotics and transcriptional regulation. Biochim Biophys Acta 1619: 263–268.
Miyazono K, Suzuki H, Imamura T . (2003). Regulation of TGF-beta signaling and its roles in progression of tumors. Cancer Sci 94: 230–234.
Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, Poellinger L et al. (2004). A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res 64: 4707–4710.
Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL et al. (2004). Genetic pathways to glioblastoma: a population-based study. Cancer Res 64: 6892–6899.
Ohtake F, Baba A, Fujii-Kuriyama Y, Kato S . (2008). Intrinsic AhR function underlies cross-talk of dioxins with sex hormone signalings. Biochem Biophys Res Commun 370: 541–546.
Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H et al. (2007). Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature 446: 562–566.
Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K et al. (2003). Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature 423: 545–550.
Poland A, Glover E, Kende AS . (1976). Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem 251: 4936–4946.
Poland A, Knutson JC . (1982). Response of murine epidermis to 2,3,7,8-tetrachlorodibenzo-p-dioxin: interaction of the ah and hr loci. Annu Rev Pharmacol Toxicol 22: 517–554.
Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E et al. (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65–71.
Santiago-Josefat B, Mulero-Navarro S, Dallas SL, Fernandez-Salguero PM . (2004). Overexpression of latent transforming growth factor-beta binding protein 1 (LTBP-1) in dioxin receptor-null mouse embryo fibroblasts. J Cell Sci 117: 849–859.
Schittenhelm J, Mittelbronn M, Nguyen TD, Meyermann R, Beschorner R . (2008). WT1 expression distinguishes astrocytic tumor cells from normal and reactive astrocytes. Brain Pathol 18: 344–353.
Seoane J, Le HV, Shen L, Anderson SA, Massague J . (2004). Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117: 211–223.
Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J et al. (2000). Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 97: 779–782.
Tritschler I, Gramatzki D, Capper D, Mittelbronn M, Meyermann R, Saharinen J et al. (2009). Modulation of TGF-beta activity by latent TGF-beta-binding protein 1 in human malignant glioma cells. Int J Cancer (in press).
Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R et al. (2004). SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res 64: 7954–7961.
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC et al. (2008). The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453: 106–109.
Weller M, Fontana A . (1995). The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res Brain Res Rev 21: 128–151.
Wick W, Platten M, Weller M . (2001). Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol 53: 177–185.
Wolff S, Harper PA, Wong JM, Mostert V, Wang Y, Abel J . (2001). Cell-specific regulation of human aryl hydrocarbon receptor expression by transforming growth factor-beta(1). Mol Pharmacol 59: 716–724.
Zaher H, Fernandez-Salguero PM, Letterio J, Sheikh MS, Fornace Jr AJ, Roberts AB et al. (1998). The involvement of aryl hydrocarbon receptor in the activation of transforming growth factor-beta and apoptosis. Mol Pharmacol 54: 313–321.
Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B et al. (1998). Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1: 611–617.
Zudaire E, Cuesta N, Murty V, Woodson K, Adams L, Gonzalez N et al. (2008). The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. J Clin Invest 118: 640–650.
Acknowledgements
We are indebted to Dr Gabrielle Haas for support with confocal microscopy. This study was supported by grants from the Jacqueline Seroussi Memorial Foundation for Cancer Research and the Swiss National Fund (NCCR Neuro) to MW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gramatzki, D., Pantazis, G., Schittenhelm, J. et al. Aryl hydrocarbon receptor inhibition downregulates the TGF-β/Smad pathway in human glioblastoma cells. Oncogene 28, 2593–2605 (2009). https://doi.org/10.1038/onc.2009.104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.104
Keywords
This article is cited by
-
The aryl hydrocarbon receptor and the gut–brain axis
Cellular & Molecular Immunology (2021)
-
Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas
Nature Cancer (2021)
-
Environmental chemicals, breast cancer progression and drug resistance
Environmental Health (2020)
-
The AHR pathway represses TGFβ-SMAD3 signalling and has a potent tumour suppressive role in SHH medulloblastoma
Scientific Reports (2020)
-
NHC-gold compounds mediate immune suppression through induction of AHR-TGFβ1 signalling in vitro and in scurfy mice
Communications Biology (2020)