Abstract
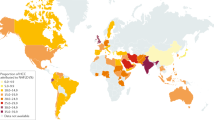
Caloric excess and sedentary lifestyle have led to a global epidemic of obesity and metabolic syndrome. The hepatic consequence of metabolic syndrome and obesity, nonalcoholic fatty liver disease (NAFLD), is estimated to affect up to one-third of the adult population in many developed and developing countries. This spectrum of liver disease ranges from simple steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis. Owing to the high prevalence of NAFLD, especially in industrialized countries but also worldwide, and the consequent burden of progressive liver disease, there is mounting epidemiological evidence that NAFLD has rapidly become a leading aetiology underlying many cases of hepatocellular carcinoma (HCC). In this Review, we discuss NAFLD-associated HCC, including its epidemiology, the key features of the hepatic NAFLD microenvironment (for instance, adaptive and innate immune responses) that promote hepatocarcinogenesis and the management of HCC in patients with obesity and associated metabolic comorbidities. The challenges and future directions of research will also be discussed, including clinically relevant biomarkers for early detection, treatment stratification and monitoring as well as approaches to therapies for both prevention and treatment in those at risk or presenting with NAFLD-associated HCC.
Key points
-
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of chronic liver disease that ranges from simple steatosis to nonalcoholic steatohepatitis (NASH) and is strongly associated with metabolic syndrome.
-
NAFLD dramatically increases the prevalence of hepatocellular carcinoma (HCC) development; however, the increased HCC risk of patients with NAFLD is often misdiagnosed.
-
The degree of fibrosis is considered the strongest predictive factor for correlating the progression of NAFLD with life-threating complications.
-
Several factors contribute to the development of NAFLD or NASH and subsequent HCC development; these factors include genetic and environmental modifiers such as diet or lifestyle.
-
The pathogenesis of NAFLD-associated HCC is a complex landscape composed of mechanisms involved in immune and inflammatory responses, DNA damage, oxidative stress and autophagy.
-
Currently, the diagnosis of NAFLD-associated HCC depends on imaging, whereas proper HCC staging is necessary for the evaluation of prognosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Swinburn, B. A. et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378, 804–814 (2011).
World Health Organization. Obesity and overweight. WHO https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2017).
Van Gaal, L. F., Mertens, I. L. & De Block, C. E. Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880 (2006).
Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66, 1138–1153 (2017).
Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018).
Bhaskaran, K. et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet 384, 755–765 (2014).
Gupta, A. et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. Am. J. Clin. Oncol. 41, 874–881 (2018).
Kanwal, F. et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 155, 1828–1837 (2018).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Burt, A. D., Lackner, C. & Tiniakos, D. G. Diagnosis and assessment of NAFLD: definitions and histopathological classification. Semin. Liver Dis. 35, 207–220 (2015).
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55, 2005–2023 (2012).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 (2013).
Dixon, J. B., Bhathal, P. S. & O’Brien, P. E. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 121, 91–100 (2001).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904 (2018).
Hardy, T., Oakley, F., Anstee, Q. M. & Day, C. P. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu. Rev. Pathol. 11, 451–496 (2016).
Day, C. P. & James, O. F. Steatohepatitis: a tale of two “hits”? Gastroenterology 114, 842–845 (1998).
Day, C. P. & Saksena, S. Non-alcoholic steatohepatitis: definitions and pathogenesis. J. Gastroenterol. Hepatol. 17 (Suppl. 3), S377–S384 (2002).
Buzzetti, E., Pinzani, M. & Tsochatzis, E. A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65, 1038–1048 (2016).
Anstee, Q. M. & Day, C. P. The genetics of nonalcoholic fatty liver disease: spotlight on PNPLA3 and TM6SF2. Semin. Liver Dis. 35, 270–290 (2015).
Utzschneider, K. M. & Kahn, S. E. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 91, 4753–4761 (2006).
Krenkel, O. et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 67, 1270–1283 (2018).
Gadd, V. L. et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 59, 1393–1405 (2014).
Moylan, C. A. et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology 59, 471–482 (2014).
Boyle, M., Masson, S. & Anstee, Q. M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J. Hepatol. 68, 251–267 (2018).
Hart, C. L., Morrison, D. S., Batty, G. D., Mitchell, R. J. & Smith, G. D. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 340, c1240 (2010).
White, D. L., Kanwal, F. & El-Serag, H. B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 10, 1342–1359 (2012).
Wong, V. W. et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 59, 969–974 (2010).
McPherson, S. et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 62, 1148–1155 (2015).
Pais, R. et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 59, 550–556 (2013).
Singh, S. et al. Fibrosis progression in nonalcoholic fatty liver versus nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 13, 643–654 (2015).
Prashanth, M. et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J. Assoc. Physicians India 57, 205–210 (2009).
Wolf, M. J. et al. Metabolic activation of intrahepatic CD8+T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26, 549–564 (2014).
Ringelhan, M., Pfister, D., O’Connor, T., Pikarsky, E. & Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 19, 222–232 (2018).
Younossi, Z. M. et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology 53, 1874–1882 (2011).
Ekstedt, M. et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397 (2015).
Dulai, P. S. et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 65, 1557–1565 (2017).
Vilar-Gomez, E. et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease. Gastroenterology 155, 443–457 (2018).
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Seyda Seydel, G. et al. Economic growth leads to increase of obesity and associated hepatocellular carcinoma in developing countries. Ann. Hepatol. 15, 662–672 (2016).
Powell, E. E. et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology 11, 74–80 (1990).
Ascha, M. S. et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 51, 1972–1978 (2010).
Sanyal, A., Poklepovic, A., Moyneur, E. & Barghout, V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 26, 2183–2191 (2010).
Baffy, G., Brunt, E. M. & Caldwell, S. H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J. Hepatol. 56, 1384–1391 (2012).
Wong, R. J., Cheung, R. & Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the US. Hepatology 59, 2188–2195 (2014).
Kim, G. A. et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 68, 140–146 (2017).
Kawamura, Y. et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am. J. Gastroenterol. 107, 253–261 (2012).
Adams, L. A. et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129, 113–121 (2005).
Petrick, J. L. et al. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int. J. Cancer 139, 1534–1545 (2016).
Bray, F. et al. Cancer Incidence in Five Continents Vol. XI (International Agency for Research on Cancer, 2017).
Dyson, J. et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 60, 110–117 (2014).
Pais, R. et al. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment. Pharmacol. Ther. 46, 856–863 (2017).
Piscaglia, F. et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 63, 827–838 (2016).
Ertle, J. et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer 128, 2436–2443 (2011).
Yasui, K. et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 9, 428–433 (2011).
Reeves, H. L., Zaki, M. Y. & Day, C. P. Hepatocellular carcinoma in obesity, type 2 diabetes, and NAFLD. Dig. Dis. Sci. 61, 1234–1245 (2016).
Baffy, G. Hepatocellular carcinoma in obesity: finding a needle in the haystack? Adv. Exp. Med. Biol. 1061, 63–77 (2018).
Mantovani, A. & Targher, G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann. Transl Med. 5, 270 (2017).
Margini, C. & Dufour, J. F. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 36, 317–324 (2016).
Teufel, A. et al. Comparison of gene expression patterns between mouse models of nonalcoholic fatty liver disease and liver tissues from patients. Gastroenterology 151, 513–525 (2016).
Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 943162 (2014).
Must, A. et al. The disease burden associated with overweight and obesity. JAMA 282, 1523–1529 (1999).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
Valenti, L. et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 51, 1209–1217 (2010).
Kozlitina, J. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46, 352–356 (2014).
Dongiovanni, P. et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 61, 506–514 (2015).
Liu, Y. L. et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 5, 4309 (2014).
Anstee, Q. M., Seth, D. & Day, C. P. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology 150, 1728–1744 (2016).
BasuRay, S., Smagris, E., Cohen, J. C. & Hobbs, H. H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 66, 1111–1124 (2017).
Ehrhardt, N. et al. Hepatic Tm6sf2 overexpression affects cellular ApoB-trafficking, plasma lipid levels, hepatic steatosis and atherosclerosis. Hum. Mol. Genet. 26, 2719–2731 (2017).
Liu, Y. L. et al. Carriage of the PNPLA3 rs738409 C>G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 61, 75–81 (2014).
Singal, A. G. et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am. J. Gastroenterol. 109, 325–334 (2014).
Seko, Y. et al. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol. Res. 47, 1083–1092 (2017).
Donati, B. et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 7, 4492 (2017).
Stickel, F. et al. Genetic variants in PNPLA3 and TM6SF2 predispose to the development of hepatocellular carcinoma in individuals with alcohol-related cirrhosis. Am. J. Gastroenterol. 113, 1475–1483 (2018).
Zucman-Rossi, J., Villanueva, A., Nault, J. C. & Llovet, J. M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149, 1226–1239 (2015).
Kechagias, S. et al. Fast-food-based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut 57, 649–654 (2008).
Leslie, T. et al. Survey of health status, nutrition and geography of food selection of chronic liver disease patients. Ann. Hepatol. 13, 533–540 (2014).
Valtueña, S. et al. Dietary glycemic index and liver steatosis. Am. J. Clin. Nutr. 84, 136–142 (2006).
Rietman, A., Sluik, D., Feskens, E. J. M., Kok, F. J. & Mensink, M. Associations between dietary factors and markers of NAFLD in a general Dutch adult population. Eur. J. Clin. Nutr. 72, 117–123 (2018).
Jensen, T. et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J. Hepatol. 68, 1063–1075 (2018).
Lanaspa, M. A. et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl Acad. Sci. USA 115, 3138–3143 (2018).
McCarthy, E. M. & Rinella, M. E. The role of diet and nutrient composition in nonalcoholic fatty liver disease. J. Acad. Nutr. Diet. 112, 401–409 (2012).
Gerber, L. et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment. Pharmacol. Ther. 36, 772–781 (2012).
Hallsworth, K. et al. Non-alcoholic fatty liver disease is associated with higher levels of. Frontline Gastroenterol. 6, 44–51 (2015).
Sunny, N. E., Bril, F. & Cusi, K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol. Metab. 28, 250–260 (2017).
Nakagawa, H. et al. Lipid metabolic reprogramming in hepatocellular carcinoma. Cancers (Basel) 10, 447 (2018).
Nishida, N. et al. Unique features associated with hepatic oxidative DNA damage and DNA methylation in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 31, 1646–1653 (2016).
Seki, S. et al. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 37, 56–62 (2002).
Tanaka, S. et al. Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J. Gastroenterol. 48, 1249–1258 (2013).
Begriche, K., Massart, J., Robin, M. A., Bonnet, F. & Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 58, 1497–1507 (2013).
Masarone, M. et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2018, 9547613 (2018).
Perla, F. M., Prelati, M., Lavorato, M., Visicchio, D. & Anania, C. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease. Children (Basel) 4, (E46 (2017).
Wiseman, H. & Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 313, 17–29 (1996).
Kuper, H., Adami, H. O. & Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 248, 171–183 (2000).
Ohnishi, S. et al. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid. Med. Cell. Longev. 2013, 387014 (2013).
Wilson, C. L. et al. NFkappaB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat. Commun. 6, 6818 (2015).
Yuan, D. et al. Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell 31, 771–789 (2017).
Canli, Ö. et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell 32, 869–883 (2017).
Teoh, N. C. et al. Defective DNA strand break repair causes chromosomal instability and accelerates liver carcinogenesis in mice. Hepatology 47, 2078–2088 (2008).
Delire, B. & Stärkel, P. The Ras/MAPK pathway and hepatocarcinoma: pathogenesis and therapeutic implications. Eur. J. Clin. Invest. 45, 609–623 (2015).
Theurillat, J. P. et al. URI is an oncogene amplified in ovarian cancer cells and is required for their survival. Cancer Cell 19, 317–332 (2011).
Tummala, K. S. et al. Inhibition of de novo NAD+ synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell 26, 826–839 (2014).
Gomes, A. L. et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell 30, 161–175 (2016).
Boege, Y. et al. A dual role of caspase-8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development. Cancer Cell 32, 342–359 (2017).
Lin, Z. et al. Prognostic value of DNA repair based stratification of hepatocellular carcinoma. Sci. Rep. 6, 25999 (2016).
Daugherity, E. K. et al. The DNA damage checkpoint protein ATM promotes hepatocellular apoptosis and fibrosis in a mouse model of non-alcoholic fatty liver disease. Cell Cycle 11, 1918–1928 (2012).
McKinnon, P. J. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu. Rev. Pathol. 7, 303–321 (2012).
Ditch, S. & Paull, T. T. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem. Sci. 37, 15–22 (2012).
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D. & Paull, T. T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Gao, D. et al. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1070–1077 (2004).
Schults, M. A. et al. Decreased nucleotide excision repair in steatotic livers associates with myeloperoxidase-immunoreactivity. Mutat. Res. 736, 75–81 (2012).
Collis, S. J., DeWeese, T. L., Jeggo, P. A. & Parker, A. R. The life and death of DNA-PK. Oncogene 24, 949–961 (2005).
Wong, R. H. et al. A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell 136, 1056–1072 (2009).
Fautrel, A. et al. Overexpression of the two nucleotide excision repair genes ERCC1 and XPC in human hepatocellular carcinoma. J. Hepatol. 43, 288–293 (2005).
Cornell, L. et al. DNA-PK-A candidate driver of hepatocarcinogenesis and tissue biomarker that predicts response to treatment and survival. Clin. Cancer Res. 21, 925–933 (2015).
Evert, M. et al. Deregulation of DNA-dependent protein kinase catalytic subunit contributes to human hepatocarcinogenesis development and has a putative prognostic value. Br. J. Cancer 109, 2654–2664 (2013).
Pascale, R. M. et al. DNA-PKcs: a promising therapeutic target in human hepatocellular carcinoma? DNA Repair (Amst.) 47, 12–20 (2016).
Pfeifer, U. Inverted diurnal rhythm of cellular autophagy in liver cells of rats fed a single daily meal. Virchows Arch. B Cell Pathol. 10, 1–3 (1972).
Khaminets, A., Behl, C. & Dikic, I. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 26, 6–16 (2016).
Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Taniguchi, K., Yamachika, S., He, F. & Karin, M. p62/SQSTM1-Dr. Jekyll and Mr. Hyde that prevents oxidative stress but promotes liver cancer. FEBS Lett. 590, 2375–2397 (2016).
Ichimura, Y. et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell 51, 618–631 (2013).
Taguchi, K., Motohashi, H. & Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16, 123–140 (2011).
Blommaart, E. F., Krause, U., Schellens, J. P., Vreeling-Sindelarova, H. & Meijer, A. J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 243, 240–246 (1997).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Tanaka, S. et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 64, 1994–2014 (2016).
Ramos-Gomez, M. et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl Acad. Sci. USA 98, 3410–3415 (2001).
Guichard, C. et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 44, 694–698 (2012).
Totoki, Y. et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 46, 1267–1273 (2014).
Schulze, K. et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 47, 505–511 (2015).
Zavattari, P. et al. Nrf2, but not beta-catenin, mutation represents an early event in rat hepatocarcinogenesis. Hepatology 62, 851–862 (2015).
Petrelli, A. et al. MicroRNA/gene profiling unveils early molecular changes and nuclear factor erythroid related factor 2 (NRF2) activation in a rat model recapitulating human hepatocellular carcinoma (HCC). Hepatology 59, 228–241 (2014).
Kim, H. et al. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology 54, 1707–1717 (2011).
Govaere, O. et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut 63, 674–685 (2014).
Govaere, O. et al. Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J. Hepatol. 64, 609–617 (2016).
Umemura, A. et al. p62, upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell 29, 935–948 (2016).
Xu, L. Z. et al. p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA. Oncogene 36, 304–317 (2017).
Yu, L. et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304, 1500–1502 (2004).
Takamura, A. et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 25, 795–800 (2011).
Nakagawa, H. et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26, 331–343 (2014).
Puri, P. et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134, 568–576 (2008).
Ley, R. E. et al. Obesity alters gut microbial ecology. Proc. Natl Acad. Sci. USA 102, 11070–11075 (2005).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Turnbaugh, P. J., Bäckhed, F., Fulton, L. & Gordon, J. I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 (2008).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Murphy, E. F. et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 62, 220–226 (2013).
Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 (2012).
Larsen, N. et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLOS ONE 5, e9085 (2010).
Boursier, J. & Diehl, A. M. Implication of gut microbiota in nonalcoholic fatty liver disease. PLOS Pathog. 11, e1004559 (2015).
Zhu, L. et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57, 601–609 (2013).
Mouzaki, M. et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLOS ONE 11, e0151829 (2016).
Llopis, M. et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 65, 830–839 (2016).
Le Roy, T. et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 62, 1787–1794 (2013).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Miele, L. et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49, 1877–1887 (2009).
Volynets, V. et al. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 57, 1932–1941 (2012).
Luther, J. et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell. Mol. Gastroenterol. Hepatol. 1, 222–232 (2015).
Kolodziejczyk, A. A., Zheng, D., Shibolet, O. & Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 11, e9302 (2018).
Gäbele, E. et al. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J. Hepatol. 55, 1391–1399 (2011).
Chavez-Talavera, O., Tailleux, A., Lefebvre, P. & Staels, B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152, 1679–1694 (2017).
Swann, J. R. et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4523–4530 (2011).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
Lefebvre, P., Cariou, B., Lien, F., Kuipers, F. & Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89, 147–191 (2009).
Wahlström, A. et al. Induction of farnesoid X receptor signaling in germ-free mice colonized with a human microbiota. J. Lipid Res. 58, 412–419 (2017).
Sinal, C. J. et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744 (2000).
Seyer, P. et al. Hepatic glucose sensing is required to preserve β cell glucose competence. J. Clin. Invest. 123, 1662–1676 (2013).
Claudel, T., Staels, B. & Kuipers, F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler. Thromb. Vasc. Biol. 25, 2020–2030 (2005).
Matsubara, T., Li, F. & Gonzalez, F. J. FXR signaling in the enterohepatic system. Mol. Cell Endocrinol. 368, 17–29 (2013).
Li, G. & Guo, G. L. Farnesoid X receptor, the bile acid sensing nuclear receptor, in liver regeneration. Acta Pharm. Sin. B 5, 93–98 (2015).
Zhu, Y., Liu, H., Zhang, M. & Guo, G. L. Fatty liver diseases, bile acids, and FXR. Acta Pharm. Sin. B 6, 409–412 (2016).
Li, G. & Guo, G. L. Role of class II nuclear receptors in liver carcinogenesis. Anticancer Agents Med. Chem. 11, 529–542 (2011).
Wagner, M., Zollner, G. & Trauner, M. Nuclear receptors in liver disease. Hepatology 53, 1023–1034 (2011).
Armstrong, L. E. & Guo, G. L. Role of FXR in liver inflammation during nonalcoholic steatohepatitis. Curr. Pharmacol. Rep. 3, 92–100 (2017).
Forman, B. M. et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81, 687–693 (1995).
Li, F. et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 4, 2384 (2013).
Jiang, C. et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Jiang, C. et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 125, 386–402 (2015).
Parséus, A. et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 66, 429–437 (2017).
Tremaroli, V. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22, 228–238 (2015).
Ryan, K. K. et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188 (2014).
Massafra, V. & van Mil, S. W. C. Farnesoid X receptor: a “homeostat” for hepatic nutrient metabolism. Biochim. Biophys. Acta 1864, 45–59 (2018).
Stanimirov, B., Stankov, K. & Mikov, M. Pleiotropic functions of bile acids mediated by the farnesoid X receptor. Acta Gastroenterol. Belg. 75, 389–398 (2012).
Mudaliar, S. et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582 (2013).
Prawitt, J. et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60, 1861–1871 (2011).
Zhang, Y. et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl Acad. Sci. USA 103, 1006–1011 (2006).
Fang, S. et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 21, 159–165 (2015).
Watanabe, M. et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J. Biol. Chem. 286, 26913–26920 (2011).
Fiorucci, S., Zampella, A. & Distrutti, E. Development of FXR, PXR and CAR agonists and antagonists for treatment of liver disorders. Curr. Top. Med. Chem. 12, 605–624 (2012).
Wang, X. et al. Bile acid receptors and liver cancer. Curr. Pathobiol Rep. 1, 29–35 (2013).
Chen, T. et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol. Cell Proteomics https://doi.org/10.1074/mcp.M110.004945 (2011).
Jansen, P. L. Endogenous bile acids as carcinogens. J. Hepatol. 47, 434–435 (2007).
Chiang, J. Y. L. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 1, 3–9 (2017).
Knisely, A. S. et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44, 478–486 (2006).
Yang, F. et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 67, 863–867 (2007).
Degirolamo, C. et al. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology 61, 161–170 (2015).
Calle, E. E. Obesity and cancer. BMJ 335, 1107–1108 (2007).
Tuncman, G. et al. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 103, 10741–10746 (2006).
Park, J., Morley, T. S., Kim, M., Clegg, D. J. & Scherer, P. E. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 10, 455–465 (2014).
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Rakhra, K. et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 18, 485–498 (2010).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Ma, C. et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257 (2016).
Fu, J. et al. Increased regulatory T cells correlate with CD8 T cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132, 2328–2339 (2007).
Gao, Q. et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 25, 2586–2593 (2007).
Hoechst, B. et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50, 799–807 (2009).
Kubes, P. & Mehal, W. Z. Sterile inflammation in the liver. Gastroenterology 143, 1158–1172 (2012).
Davis, B. K., Wen, H. & Ting, J. P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 (2011).
Arrese, M., Cabrera, D., Kalergis, A. M. & Feldstein, A. E. Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 61, 1294–1303 (2016).
Feldstein, A. E. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin. Liver Dis. 30, 391–401 (2010).
Peverill, W., Powell, L. W. & Skoien, R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int. J. Mol. Sci. 15, 8591–8638 (2014).
Lanthier, N. Targeting Kupffer cells in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: why and how? World J. Hepatol. 7, 2184–2188 (2015).
Reid, D. T. et al. Kupffer cells undergo fundamental changes during the development of experimental NASH and are critical in initiating liver damage and inflammation. PLOS ONE 11, e0159524 (2016).
Bieghs, V. & Trautwein, C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol. 34, 446–452 (2013).
Miura, K. et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139, 323–334 (2010).
Wu, J. et al. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 72, 3977–3986 (2012).
Ju, C. & Tacke, F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell. Mol. Immunol. 13, 316–327 (2016).
Malehmir, M. et al. Platelet GPIba is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. (in the press).
Kurien, B. T. & Scofield, R. H. Autoimmunity and oxidatively modified autoantigens. Autoimmun. Rev. 7, 567–573 (2008).
Nobili, V. et al. Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int. J. Mol. Med. 26, 471–476 (2010).
DeFuria, J. et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T cell function and an inflammatory cytokine profile. Proc. Natl Acad. Sci. USA 110, 5133–5138 (2013).
McPherson, S., Henderson, E., Burt, A. D., Day, C. P. & Anstee, Q. M. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J. Hepatol. 60, 1055–1062 (2014).
Shalapour, S. et al. Inflammation-induced IgA+cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371 (2017).
Matter, M. S., Decaens, T., Andersen, J. B. & Thorgeirsson, S. S. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J. Hepatol. 60, 855–865 (2014).
Guri, Y., Nordmann, T. M. & Roszik, J. mTOR at the transmitting and receiving ends in tumor immunity. Front. Immunol. 9, 578 (2018).
Hagiwara, A. et al. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 15, 725–738 (2012).
Guri, Y. et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell 32, 807–823 (2017).
European Association for the Study of the Liver & European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 56, 908–943 (2012).
Heimbach, J. K. et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 67, 358–380 (2017).
Taylor, E. J., Jones, R. L., Guthrie, J. A. & Rowe, I. A. Modeling the benefits and harms of surveillance for hepatocellular carcinoma: Information to support informed choices. Hepatology 66, 1546–1555 (2017).
Singal, A. G. et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev. Res. (Phila.) 5, 1124–1130 (2012).
Mittal, S. et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin. Gastroenterol. Hepatol. 13, 594–601 (2015).
Della Corte, C. & Colombo, M. Surveillance for hepatocellular carcinoma. Semin. Oncol. 39, 384–398 (2012).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 59, 1121–1140 (2016).
Stickel, F. & Hellerbrand, C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut 59, 1303–1307 (2010).
Morling, J. R. et al. Clinically significant chronic liver disease in people with type 2 diabetes: the Edinburgh type 2 diabetes study. QJM 109, 249–256 (2016).
Wen, C. P. et al. Hepatocellular carcinoma risk prediction model for the general population: the predictive power of transaminases. J. Natl Cancer Inst. 104, 1599–1611 (2012).
Berhane, S. et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin. Gastroenterol. Hepatol. 14, 875–886 (2016).
Hwang, A. et al. Supervised learning reveals circulating biomarker levels diagnostic of hepatocellular carcinoma in a clinically relevant model of non-alcoholic steatohepatitis; an OAD to NASH. PLOS ONE 13, e0198937 (2018).
Ng, C. K. Y., Di Costanzo, G. G., Terracciano, L. M. & Piscuoglio, S. Circulating cell-free DNA in hepatocellular carcinoma: current insights and outlook. Front. Med. (Lausanne) 5, 78 (2018).
Zhang, Y. J. et al. Predicting hepatocellular carcinoma by detection of aberrant promoter methylation in serum DNA. Clin. Cancer Res. 13, 2378–2384 CCR-06-1900 (2007).
Tangkijvanich, P. et al. Serum LINE-1 hypomethylation as a potential prognostic marker for hepatocellular carcinoma. Clin. Chim. Acta 379, 127–133 (2007).
Xu, R. H. et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 16, 1155–1161 (2017).
Yáñez-Mó, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015).
Hirsova, P. et al. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology 64, 2219–2233 (2016).
Arbelaiz, A. et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 66, 1125–1143 (2017).
Torbenson, M. & Schirmacher, P. Liver cancer biopsy - back to the future?! Hepatology 61, 431–433 (2015).
Sherman, M. & Bruix, J. Biopsy for liver cancer: How to balance research needs with evidence-based clinical practice. Hepatology 61, 433–437 (2015).
Friemel, J. et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin. Cancer Res. 21, 1951–1961 (2015).
Bruix, J. et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J. Hepatol. 67, 999–1008 (2017).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Cheng, A. L. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 (2009).
Mehnert, J. M. et al. The challenge for development of valuable immuno-oncology biomarkers. Clin. Cancer Res. 23, 4970–4979 (2017).
Pantel, K. & Alix-Panabieres, C. Liquid biopsy in 2016: circulating tumour cells and cell-free DNA in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 14, 73–74 (2017).
Ogle, L. F. et al. Imagestream detection and characterisation of circulating tumour cells - A liquid biopsy for hepatocellular carcinoma? J. Hepatol. 65, 305–313 (2016).
Sun, Y. F. et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology 57, 1458–1468 (2013).
Li, J. et al. Detection of circulating tumor cells in hepatocellular carcinoma using antibodies against asialoglycoprotein receptor, carbamoyl phosphate synthetase 1 and pan-cytokeratin. PLOS ONE 9, e96185 (2014).
Kalinich, M. et al. An RNA-based signature enables high specificity detection of circulating tumor cells in hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 114, 1123–1128 (2017).
Morris, K. L. et al. Circulating biomarkers in hepatocellular carcinoma. Cancer Chemother. Pharmacol. 74, 323–332 (2014).
Sun, Y. F. et al. Circulating tumor cells from different vascular sites exhibit spatial heterogeneity in epithelial and mesenchymal composition and distinct clinical significance in hepatocellular carcinoma. Clin. Cancer Res. 24, 547–559 (2018).
Li, J. et al. pERK/pAkt phenotyping in circulating tumor cells as a biomarker for sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncotarget 7, 2646–2659 (2016).
Lorentzen, A. et al. Single cell polarity in liquid phase facilitates tumour metastasis. Nat. Commun. 9, 887 (2018).
Prentis, J. M. et al. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl. 18, 152–159 (2012).
Kolly, P. et al. Assessment of the Hong Kong Liver Cancer Staging System in Europe. Liver Int. 36, 911–917 (2016).
Kadalayil, L. et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 24, 2565–2570 (2013).
Sieghart, W. et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 57, 2261–2273 (2013).
Varela, M. et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J. Hepatol. 46, 474–481 (2007).
Vilgrain, V. et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 18, 1624–1636 (2017).
Kirstein, M. M. et al. Patterns and challenges of treatment sequencing in patients with hepatocellular carcinoma: experience from a German referral center. J. Gastroenterol. Hepatol. 32, 1730–1738 (2017).
Pardo, F. et al. The Post-SIR-Spheres Surgery Study (P4S): retrospective analysis of safety following hepatic resection or transplantation in patients previously treated with selective internal radiation therapy with Yttrium-90 resin microspheres. Ann. Surg. Oncol. 24, 2465–2473 (2017).
Moir, J. A. et al. Selective internal radiation therapy for liver malignancies. Br. J. Surg. 102, 1533–1540 (2015).
Kudo, M. Lenvatinib in advanced hepatocellular carcinoma. Liver Cancer 6, 253–263 (2017).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Kudo, M. Immune checkpoint blockade in hepatocellular carcinoma. Liver Cancer 4, 201–207 (2015).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Raoul, J. L. et al. Systemic therapy for intermediate and advanced hepatocellular carcinoma: Sorafenib and beyond. Cancer Treat. Rev. 68, 16–24 (2018).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378 (2015).
Mishra, S. I. et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 8, CD007566 (2012).
Mishra, S. I. et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst. Rev. 8, CD008465 (2012).
Gustafson, M. P. et al. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J. Immunother. Cancer 5, 30 (2017).
Idorn, M. & Thor Straten, P. Exercise: a new role for an old tool. Mol. Cell Oncol. 3, e1163005 (2016).
Koelwyn, G. J., Quail, D. F., Zhang, X., White, R. M. & Jones, L. W. Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer 17, 620–632 (2017).
McCuskey, R. S. et al. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology 40, 386–393 (2004).
Iyer, S., Upadhyay, P. K., Majumdar, S. S. & Nagarajan, P. Animal models correlating immune cells for the development of NAFLD/NASH. J. Clin. Exp. Hepatol. 5, 239–245 (2015).
Denda, A. et al. Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn J. Cancer Res. 93, 125–132 (2002).
Matsumoto, M. et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 94, 93–103 (2013).
Ikawa-Yoshida, A. et al. Hepatocellular carcinoma in a mouse model fed a choline-deficient, L-amino acid-defined, high-fat diet. Int. J. Exp. Pathol. 98, 221–233 (2017).
Sun, B. & Karin, M. Obesity, inflammation, and liver cancer. J. Hepatol. 56, 704–713 (2012).
Charlton, M. et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G825–G834 (2011).
Asgharpour, A. et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 65, 579–588 (2016).
Wang, Y., Ausman, L. M., Greenberg, A. S., Russell, R. M. & Wang, X. D. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int. J. Cancer 124, 540–546 (2009).
Tsuchida, T. et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 69, 385–395 (2018).
Acknowledgements
Q.M.A., O.G. and H.L.R. are members of the EPoS (Elucidating Pathways of Steatohepatitis) consortium funded by the Horizon 2020 Framework Programme of the European Union under Grant Agreement 634413 and the Newcastle National Institute for Health Research Biomedical Research Centre. At the Northern Institute for Cancer Research, H.L.R. is supported by the Bobby Robson Foundation, Cancer Research UK (CRUK) Newcastle Experimental Cancer Medicine Centre award C9380/A18084 and CRUK programme grant C18342/A23390. This work was supported by the European Union’s Horizon 2020 Research and Innovation Programme (no. 667273/HEPCAR) to M.H.; a European Research Council Consolidator grant ‘HepatoMetaboPath’; the MOST (Ministry of Science and Technology) programme; Research Foundation Flanders (FWO) under grant 30826052 (EOS Convention MODEL-IDI); the Sonderforschungsbereiche (SFB) Transregio (TR) SFB/TR179, 209 and SFB1335 to M.H.; Graduiertenkolleg (GRK482) to E.K. and M.H.; and the German Cancer Research Center (DKFZ)–MOST cooperation program to M.H.
Reviewer information
Nature Reviews Gastroenterology & Hepatology thanks M. Machado and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anstee, Q.M., Reeves, H.L., Kotsiliti, E. et al. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 16, 411–428 (2019). https://doi.org/10.1038/s41575-019-0145-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-019-0145-7
This article is cited by
-
Phosphorylation: new star of pathogenesis and treatment in steatotic liver disease
Lipids in Health and Disease (2024)
-
Unraveling the underlying pathogenic factors driving nonalcoholic steatohepatitis and hepatocellular carcinoma: an in-depth analysis of prognostically relevant gene signatures in hepatocellular carcinoma
Journal of Translational Medicine (2024)
-
MyD88 in myofibroblasts enhances nonalcoholic fatty liver disease-related hepatocarcinogenesis via promoting macrophage M2 polarization
Cell Communication and Signaling (2024)
-
Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies
Signal Transduction and Targeted Therapy (2024)
-
Perilipin 5 deletion protects against nonalcoholic fatty liver disease and hepatocellular carcinoma by modulating lipid metabolism and inflammatory responses
Cell Death Discovery (2024)