Abstract
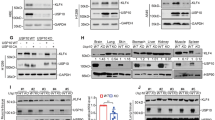
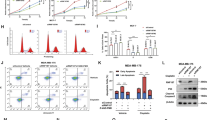
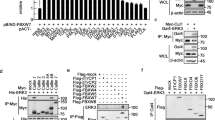
Ubiquitin-mediated proteolysis plays a central role in controlling intracellular levels of essential regulatory molecules such as p53, cyclins, myc, BRCA1, HIF-1α, etc. The Kruppel-like factor 5 (KLF5) transcription factor regulates biological processes involved in carcinogenesis, angiogenesis, and smooth muscle cell differentiation. In carcinogenesis, KLF5's role has been indicated by frequent genetic deletion as well as functional studies. Here we show that KLF5 is an unstable protein with a short half-life. Destruction of KLF5 was prevented by each of the proteasome-specific inhibitors tested but not by an inhibitor for trypsin-like proteases and cysteine proteases or by a lysosome inhibitor in epithelial cells. Furthermore, KLF5 underwent ubiquitination, and deletion of a 56-amino-acid sequence adjacent to a known transactivation domain of KLF5 significantly reduced its ubiquitination and degradation. Interestingly, cancer cells appeared to be more active in KLF5 degradation than untransformed epithelial cells, yet their proteasome activity was not higher. These results suggest that KLF5 protein is degraded at least in part through ubiquitination–proteasome pathway, which may have become hyperactive for KLF5 in cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Adam PJ, Regan CP, Hautmann MB and Owens GK . (2000). J. Biol. Chem., 275, 37798–37806.
Aizawa K, Suzuki T, Kada N, Ishihara A, Kawai-Kowase K, Matsumura T, Sasaki K, Munemasa Y, Manabe I, Kurabayashi M, Collins T and Nagai R . (2004). J. Biol. Chem., 279, 70–76.
Bateman NW, Tan D, Pestell RG, Black JD and Black AR . (2004). J. Biol. Chem., 279, 12093–12101.
Black AR, Black JD and Azizkhan-Clifford J . (2001). J. Cell. Physiol., 188, 143–160.
Brondello JM, Pouyssegur J and McKenzie FR . (1999). Science, 286, 2514–2517.
Campanero MR and Flemington EK . (1997). Proc. Natl. Acad. Sci. USA, 94, 2221–2226.
Chen C, Bhalala HV, Qiao H and Dong JT . (2002). Oncogene, 21, 6567–6572.
Chen C, Bhalala HV, Vessella RL and Dong JT . (2003). Prostate, 55, 81–88.
Chen C, Zhou Y, Zhou Z, Sun X, Otto KB, Uht RM and Dong JT . (2004). Gene, 330, 133–142.
Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D and Maniatis T . (1995). Genes Dev., 9, 1586–1597.
Dang DT, Pevsner J and Yang VW . (2000). Int. J. Biochem. Cell Biol., 32, 1103–1121.
Grinstein E, Jundt F, Weinert I, Wernet P and Royer HD . (2002). Oncogene, 21, 1485–1492.
Haupt Y, Maya R, Kazaz A and Oren M . (1997). Nature, 387, 296–299.
Herbst A, Salghetti SE, Kim SY and Tansey WP . (2004). Oncogene, 23, 3863–3871.
Huang LE, Gu J, Schau M and Bunn HF . (1998). Proc. Natl. Acad. Sci. USA, 95, 7987–7992.
Johnsen SA, Subramaniam M, Monroe DG, Janknecht R and Spelsberg TC . (2002). J. Biol. Chem., 277, 30754–30759.
Knuutila S, Aalto Y, Autio K, Bjorkqvist AM, El-Rifai W, Hemmer S, Huhta T, Kettunen E, Kiuru-Kuhlefelt S, Larramendy ML, Lushnikova T, Monni O, Pere H, Tapper J, Tarkkanen M, Varis A, Wasenius VM, Wolf M and Zhu Y . (1999). Am. J. Pathol., 155, 683–694.
Kojima S, Kobayashi A, Gotoh O, Ohkuma Y, Fujii-Kuriyama Y and Sogawa K . (1997). J. Biochem. (Tokyo), 121, 389–396.
Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM and Pagano M . (1997). Nat. Med., 3, 231–234.
Miyamoto S, Suzuki T, Muto S, Aizawa K, Kimura A, Mizuno Y, Nagino T, Imai Y, Adachi N, Horikoshi M and Nagai R . (2003). Mol. Cell. Biol., 23, 8528–8541.
Murphy TJ, Pavlath GK, Wang X, Boss V, Abbott KL, Robida AM, Nichols J, Xu K, Ellington ML and Loss II JR . (2002). Methods Enzymol., 345, 539–551.
Nandan MO, Yoon HS, Zhao W, Ouko LA, Chanchevalap S and Yang VW . (2004). Oncogene, 23, 3404–3413.
Ogata T, Kurabayashi M, Hoshino Y, Sekiguchi K, Ishikawa S, Morishita Y and Nagai R . (2000). J. Thorac. Cardiovasc. Surg., 119, 983–989.
Ohnishi S, Laub F, Matsumoto N, Asaka M, Ramirez F, Yoshida T and Terada M . (2000). Dev. Dyn., 217, 421–429.
Pickart CM . (2001). Annu. Rev. Biochem., 70, 503–533.
Rechsteiner M and Rogers SW . (1996). Trends Biochem. Sci., 21, 267–271.
Salghetti SE, Kim SY and Tansey WP . (1999). EMBO J., 18, 717–726.
Salghetti SE, Muratani M, Wijnen H, Futcher B and Tansey WP . (2000). Proc. Natl. Acad. Sci. USA, 97, 3118–3123.
Shi H, Zhang Z, Wang X, Liu S and Teng CT . (1999). Nucleic Acids Res., 27, 4807–4815.
Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M and Nagai R . (2002). Nat. Med., 8, 856–863.
Su K, Roos MD, Yang X, Han I, Paterson AJ and Kudlow JE . (1999). J. Biol. Chem., 274, 15194–15202.
Sun R, Chen X and Yang VW . (2001). J. Biol. Chem., 276, 6897–6900.
Taneyhill L and Pennica D . (2004). BMC Dev. Biol., 4, 6.
Usui S, Sugimoto N, Takuwa N, Sakagami S, Takata S, Kaneko S and Takuwa Y . (2004). J. Biol. Chem., 279, 12300–12311.
Wada Y, Suzuki J, Kawauchi M, Kurabayashi M, Tsukioka K, Zhang T, Endoh M, Takayama K, Nagai R, Takamoto S, Isobe M and Amano J . (2001). J. Heart Lung Transplant., 20, 590–594.
Wada Y, Suzuki J, Tsukioka K, Zhang T, Takayama K, Endoh M, Watanabe N, Kurabayashi M, Kawauchi M, Nagai R, Takamoto S, Isobe M and Amano J . (2000). Transplant. Proc., 32, 1089–1091.
Zhang X, Srinivasan SV and Lingrel JB . (2004). Biochem. Biophys. Res. Commun., 316, 139–148.
Zhang Z and Teng CT . (2003). Nucleic Acids Res., 31, 2196–2208.
Ziemer LT, Pennica D and Levine AJ . (2001). Mol. Cell. Biol., 21, 562–574.
Acknowledgements
We thank Dr Jun Zhou for providing the HA-Ub expression plasmid and Dr Shi-Yong Sun for valuable discussions. C Chen is an AFUD/AUA Research Scholar. This work was supported in part by NIH Grant CA87921 from the National Cancer Institute, by the Georgia Cancer Coalition, and by Grant DAMD17-03-2-0033 from the Department of Defense Prostate Cancer Research Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C., Sun, X., Ran, Q. et al. Ubiquitin–proteasome degradation of KLF5 transcription factor in cancer and untransformed epithelial cells. Oncogene 24, 3319–3327 (2005). https://doi.org/10.1038/sj.onc.1208497
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208497