-
PDF
- Split View
-
Views
-
Cite
Cite
Soichi Yamashita, Kazuto Nakamura, Yuki Omori, Katsuhiko Tsunekawa, Masami Murakami, Takashi Minegishi, Association of Human Follitropin (FSH) Receptor with Splicing Variant of Human Lutropin/Choriogonadotropin Receptor Negatively Controls the Expression of Human FSH Receptor, Molecular Endocrinology, Volume 19, Issue 8, 1 August 2005, Pages 2099–2111, https://doi.org/10.1210/me.2005-0049
Close - Share Icon Share
Abstract
A splice variant of human lutropin (LH)/choriogonadotropin (CG)-receptor [hLHR(exon 9)] that lacks exon 9 was previously cloned in the corpus luteum of a woman with a normal menstrual cycle. Supported by a detergent-soluble binding assay and a receptor biotinylation experiment, the receptor binding assay shows hLHR(exon 9) is neither expressed at the cell surface nor has the capability of binding to hCG. In addition, hLHR(exon 9) was confirmed in the endoplasmic reticulum (ER) by endoglycosidase H treatment. A coimmunoprecipitation experiment clearly showed that hLHR(exon 9) and constitutively inactivate mutant-LHRs, which stay in the ER, form an association with the human follitropin (FSH)-receptor (hFSHR). This suggests that in the presence of mutant-LHR, hFSHR, which is trapped in the ER and associated with hLHR(exon 9), is unable to come up to the plasma membrane. This phenomenon is specific among gonadotropin receptors because human TSH receptor failed to be coimmunoprecipitated. Furthermore, this receptor complex attenuated the hFSHR receptor protein level within the cells, which impaired cAMP production. To elucidate the mechanism underlying the decrease in hFSHR protein by this receptor complex, we performed a Percoll fractionation experiment, which indicated that the receptor complex drove hFSHR to the lysosome instead of the plasma membrane. These results reveal a novel mechanism of FSHR expression regulation.
THE HUMAN FSH RECEPTOR (hFSHR), found in Sertoli cells of the testes and granulosa cells of the ovary, is a member of the superfamily of G protein-coupled receptors (GPCRs) and is required for normal reproductive function in males and females. All GPCRs share a common serpentine structure consisting of seven transmembrane helices linked by three alternating intracellular and extracellular loops. The extracellular regions are involved in ligand binding, whereas the intracellular regions are primarily involved in signaling. The FSHR, along with the receptors for LH/choriogonadotropin (CG) receptor (LHR) and TSH receptor (TSHR), form a subfamily of the superfamily of GPCRs (1, 2), distinguished by their large extracellular N-terminal domain encoding almost half of the receptor. When we cloned hFSHR (3) and hLHR (4), we found the amino acid sequence identity between the hFSHR and hLHR was approximately 42% in the extracellular domain, 70% in the serpentine region, and 29% in the C-terminal cytoplasmic tail. At the same time, we also cloned a splice variant of hLHR, from the corpus luteum of a woman with a normal menstrual cycle, lacking exon 9 coding 62 amino acids [hLHR(exon 9)]. The physiological function of hLHR(exon 9) in the human ovary is not yet known. In addition, two additional deletion mutants within the N-terminal extracellular domain of hLHR (i.e. exons 8 and 10) were found (5, 6). In both cases, these naturally occurring mutations cause Leydig cell hypoplasia, resulting in complete or partial feminization of the external genitalia. Moreover, we detected three transcripts (5.4, 3.6, and 2.4 kb) for hLHR mRNA in the human ovary by Northern blot analysis, showing that both wild-type hLHR and hLHR(exon 9) are generated by alternative splicing (7). These data suggest that hLHR(exon 9) may have a specific function in the ovary.
Because reports demonstrate that many GPCRs exist as dimers or oligomers, dimerization or oligomerization of GPCRs is important for receptor function, including agonist affinity, potency, and efficacy and G protein specificity (8). A recent report describes that FSH and FSHR complexes form dimers in the crystallography (9), expanding the possibility of receptor dimers being an important factor for gonadotropin receptors. Furthermore, the discovery of heterodimerization of GPCRs complicates the understanding of the general mechanism of GPCR modulation and function. For instance, the heterodimeric κ-δ opioid receptor synergistically binds receptor-selective ligands with higher binding affinity (10). In the case of β-adrenergic receptor (βAR), the heterodimerization of β1AR and β2AR inhibits β2AR-dependent activation of the ERK1/2 MAPK signaling pathway (11). In addition, heterodimerization of type 1 somatostatin receptor (SSTR1) and SSTR5 reduces the internalization of SSTR5 (12).
The expression level of FSHR in the granulosa cells is gradually elevated toward ovulation (13) and has a critical role in stimulating follicular maturation. LHR, expressed in theca, stromal, late-stage granulosa, and luteal cells, is important in triggering ovulation and maintaining progesterone production of corpus luteum thereafter.
To date, there is no direct evidence addressing whether hFSHR and hLHR actually form a complex. To examine whether these receptors can form receptor complexes, we used immunoprecipitation of the tagged hFSHR and hLHR(exon 9), using c-Myc and FLAG respectively, in this study. To elucidate the effect of the receptor association of hFSHR with hLHR(exon 9), agonist-induced cAMP accumulation was examined. We found that hFSHR and hLHR(exon 9) can form receptor complexes: the reduction in the protein levels for hFSHR was followed by the formation of receptor complexes between hFSHR and hLHR(exon 9), which is facilitated to drive to the lysosome after the association.
RESULTS
The Association of Myc-hFSHR and FLAG-hLHR(Exon 9)
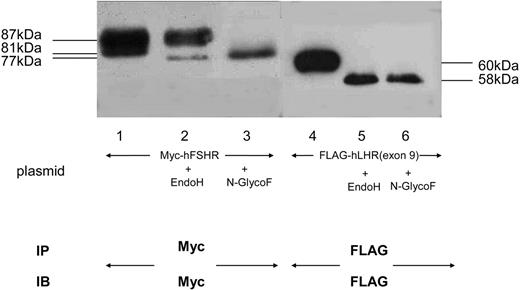
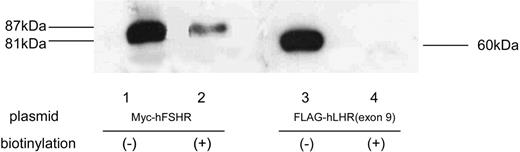
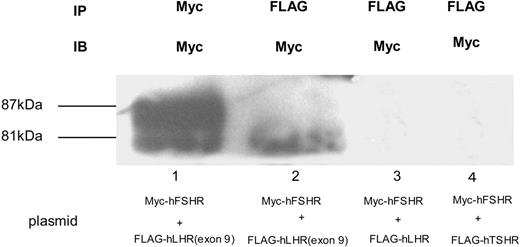
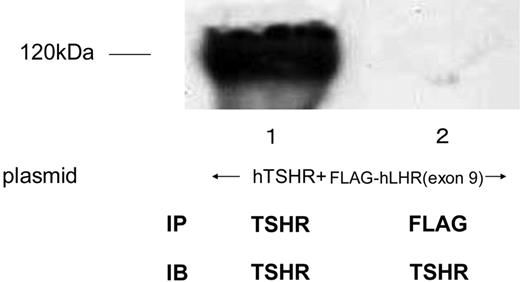
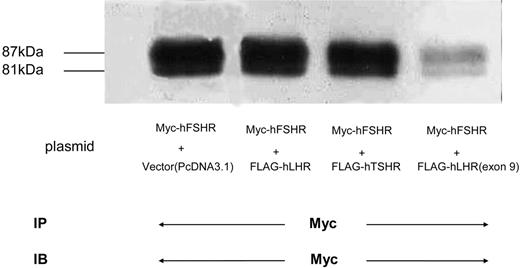
To examine whether Myc-hFSHR and FLAG-hLHR(exon 9) form complexes, we used human embryonic kidney (HEK)293 cells transiently expressing Myc-hFSHR and/or FLAG-hLHR(exon 9). Western blots of extracts of cells that had been transiently transfected with Myc-hFSHR display two bands: 87 kDa and 81 kDa (Fig. 1, lane 1). Those of FLAG-hLHR(exon 9) display one band: 60 kDa; Fig. 1, lane 4). Further experiments with endoglycosidase H (EndoH), which cleaves high mannose-containing immature N-linked carbohydrates from glycoproteins, were done to confirm the 81-kDa band of hFSHR is immature protein. As shown in Fig. 1, the 81-kDa band was sensitive to EndoH (lane 2) and underwent a shift to 77 kDa, whereas the treatment with N-glycosidase F (N-GlycoF) caused migration of both 87-kDa and 81-kDa bands to 77-kDa bands (lane 3). The 60-kDa band of FLAG-hLHR(exon 9), sensitive to both EndoH and N-GlycoF, underwent a shift to 58 kDa (Fig. 1, lanes 5 and 6). These data indicate that the 81-kDa band of hFSHR and the 60-kDa band of hLHR(exon 9) are immature proteins of hFSHR and hLHR, which still reside within the endoplasmic reticulum (ER). To confirm this, we then examined whether FLAG-hLHR(exon 9) is expressed at the cell surface. These experiments were established by the biotinylation of the cell surface proteins followed by Western blotting with horseradish peroxidase (HRP)-conjugated streptavidin. As shown in Fig. 2, streptavidin blots of anti-c-Myc immunoprecipitates of lysates obtained from biotinylated cells transfected with Myc-hFSHR resulted in the visualization of a prominent (87 kDa) band (lane 2), whereas the anti-c-Myc blot resulted in both the 87-kDa and 81-kDa bands (lane 1). On the other hand, FLAG-hLHR(exon 9) expressed alone because streptavidin blots failed to detect the 60-kDa bands of FLAG-hLHR(exon 9) (lane 4). These findings indicated that FLAG-hLHR(exon 9) was retained intracellularly. In the HEK293 cells coexpressing both Myc-hFSHR and FLAG-hLHR(exon 9), immature forms of Myc-hFSHR (81 kDa) were coprecipitated by the anti-FLAG antibody (Fig. 3 lane 2), using differential immunoprecipitation. This finding was specific: it did not occur among cells individually expressing the receptors (data not shown). On the other hand, in the HEK293 cells expressing both Myc-hFSHR and FLAG-hLHR, or both Myc-hFSHR and FLAG-hTSHR, which is categorized to a subfamily of the superfamily of GPCRs as hFSHR and hLHR, no bands were detected (Fig. 3, lanes 3 and 4). We also examined whether hTSHR and FLAG-hLHR(exon 9) form complexes. As shown in Fig. 4, hTSHR was not coprecipitated by the anti-FLAG antibody. These data indicated that the immature form of Myc-hFSHR specifically interacts with FLAG-hLHR(exon 9), but not with FLAG-hLHR or FLAG-hTSHR.
Glycosylation Condition of Myc-hFSHR and FLAG-hLHR(Exon 9) HEK293 cells expressing either Myc-hFSHR or FLAG-hLHR(exon 9) were solubilized in detergent and were incubated in the absence (−) or presence (+) of either EndoH or N-GlycoF as described in Materials and Methods. After incubation, each sample was immunoprecipitated, resolved by 7.5% reducing SDS-PAGE, transferred to a PVDF membrane, and confirmed with either anti-c-Myc or M2 anti-FLAG antibody. The blot is representative of three independent experiments. IP, Immunoprecipitation; IB, immunoblot.
Biotinylation of Myc-hFSHR and FLAG-hLHR(Exon 9) HEK293 cells were transiently transfected with Myc-hFSHR or FLAG-hLHR(exon 9). The cell surface proteins were covalently modified with biotin, and lysates were prepared, immunoprecipitated with anti-c-Myc or M2 anti-FLAG antibody, and resolved by SDS-PAGE. After electrophoretic blotting, the biotinylated proteins were visualized using HRP-labeled streptavidin and ECL-Plus as described in Materials and Methods. Myc-hFSHR or FLAG-hLHR(exon 9), which were not biotinylated, were visualized as in Fig. 1.
Detection of Myc-hFSHR Association by Immunoblotting and Coimmunoprecipitation HEK293 cells coexpressing both Myc-hFSHR and FLAG-hLHR(exon 9) (lanes 1 and 2) or both Myc-hFSHR and FLAG-hLHR (lane 3), or both Myc-hFSHR and FLAG-hTSHR (lane 4) were solubilized as described in Materials and Methods, immunoprecipitated (IP) with anti-c-Myc –antibody (lane 1) or anti-FLAG-antibody (lanes 2–4), and resolved by 7.5% reducing SDS-PAGE. After being transferred to a PVDF membrane, each sample was confirmed with anti-c-Myc-antibody (IB). The blot is representative of three independent experiments. IP, Immunoprecipitation.
Coimmunoprecipitation of hTSHR and FLAG-hLHR(Exon 9) HEK293 cells coexpressing both hTSHR and FLAG-hLHR(exon 9) were solubilized as described in Materials and Methods, immunoprecipitated (IP) with anti-c-TSHR-antibody (lane1) or anti-FLAG-antibody (lane 2), and resolved by 7.5% reducing SDS-PAGE. After being transferred to a PVDF membrane, confirmed with anti-c-TSHR-antibody (IB). The blot is representative of three independent experiments.
Coexpression of FLAG-hLHR(Exon 9) with the Wild-Type Myc-hFSHR Decreases Expression of the Wild-Type hFSHR
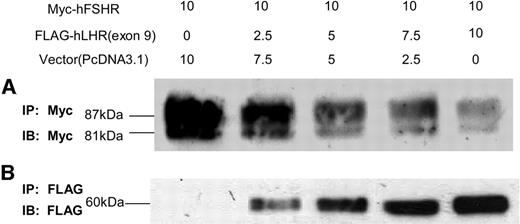
To examine whether the association of hFSHR and hLHR(exon 9) modulates the expression of the wild-type hFSHR, we cotransfected HEK293 cells with Myc-hFSHR and increasing amounts of plasmid of FLAG-hLHR(exon 9) for transfection. As shown in Fig. 5, and as was in the case with increasing expression of FLAG-hLHR(exon 9) in the HEK293 cells (Fig. 5B), both 87-kDa and 81-kDa bands of Myc-hFSHR were diminished (Fig. 5A). To confirm that this phenomenon is specific, we cotransfected HEK293 cells with Myc-hFSHR and FLAG-hLHR, or Myc-hFSHR and FLAG-hTSHR. As shown in Fig. 6, the cotransfection with FLAG-hLHR(exon 9) decreased expression of Myc-hFSHR, but FLAG-hLHR and FLAG-hTSHR did not suppress the expression of Myc-hFSHR. These experiments revealed that the suppression of Myc-hFSHR expression specifically occurred by the cotransfection with FLAG-hLHR(exon 9).
Coimmunoprecipitation of Myc-hFSHR and FLAG-hLHR(Exon 9) HEK293 cells transiently transfected with Myc-hFSHR and increasing amounts of FLAG-hLHR(exon 9). Total amount of DNA used for each transfection condition was kept constant by the addition of an appropriate amount of pcDNA3.1 vector. HEK293 cells were solubilized as described in Materials and Methods, immunoprecipitated (IP) with anti-c-Myc-antibody or anti-FLAG-antibody, and resolved by 7.5% reducing SDS-PAGE. After being transferred to a PVDF membrane, confirmed with an indicated antibody (IB). A, HEK293 cells cotransfected with Myc-hFSHR and FLAG-hLHR(exon 9) were immunoprecipitated with anti-c-Myc-antibody and immunoblotted with anti-c-Myc-antibody to detect the expression of Myc-hFSHR. B, HEK293 cells cotransfected with Myc-hFSHR and FLAG-hLHR(exon 9) were immunoprecipitated with M2 anti-FLAG antibody and immunoblotted with M2 anti-FLAG antibody to detect the expression of FLAG-hLHR(exon 9). The blot is representative of three independent experiments.
Expression of Myc-hFSHR Coexpressed with Other GPCRs HEK293 cells were transiently transfected with either Myc-hFSHR alone (lane 1) or both Myc-hFSHR and FLAG-hLHR (lane 2), Myc-hFSHR and FLAG-hTSHR (lane 3), or Myc-hFSHR and FLAG-hLHR(exon 9) (lane 4). Cells were solubilized as described in Materials and Methods, immunoprecipitated (IP) with anti-c-Myc-antibody, and resolved by 7.5% reducing SDS-PAGE. After being transferred to a PVDF membrane, confirmed with anti-c-Myc antibody to detect the expression of Myc-hFSHR. The blot is representative of three independent experiments. IB, Immunoblot.
We next examined whether cotransfection with FLAG-hLHR(exon 9) modulates the binding ability of hFSHR for hFSH. First, we examined whether FLAG-hLHR(exon 9) has binding ability. As shown in Table 1, FLAG-hLHR(exon 9) has no binding ability on the cell surface and only slight binding overall. Combined with the results from receptor biotinylation experiments (Fig. 2), these results confirm our findings that FLAG-hLHR(exon 9) exists inside the cell. Table 2 shows that the coexpression of FLAG-hLHR(exon 9) with Myc-hFSHR reduced receptor expression at the cell surface from 38,600 ± 3,500 to 25,300 ± 4,800, without changing the affinity for [125I]hFSH. We also examined whether the association of hFSHR and hLHR(exon 9) modulates the signal transduction by hFSHR. We quantitated cAMP accumulation in cells incubated with increasing concentrations of hFSH. As shown in Table 2, the basal levels of cAMP and the EC50 for the hFSH-induced cAMP production do not differ between cells expressing Myc-hFSHR alone and cells expressing both Myc-hFSHR and FLAG-hLHR(exon 9). In contrast, the maximal response in cells expressing both receptors was reduced about 50% as compared with those solely expressing Myc-hFSHR. To confirm that this phenomenon was caused by reducing receptor number, we used different amounts of Myc-hFSHR plasmid for transfection. As shown in Table 3, when the plasmid amount for transfection was reduced, the receptor expression at the cell surface and the maximal cAMP production were significantly decreased. These data indicated that the cotransfection with FLAG-hLHR(exon 9) reduces the expression of Myc-hFSHR and modulates the biological activity of Myc-hFSHR.
The Binding Activity of Receptors Expressing Cell Surface and Total Cells
| Receptor . | Intact Cells (ng/106 cells) . | Detergent Extracts (ng/106 cells) . |
|---|---|---|
| Myc-hFSHR ([125I]FSH binding) | 3.2 ± 0.13 | 3.9 ± 0.39 |
| Myc-hLHR ([125I]hCG binding) | 6.3 ± 0.68 | 11.0 ± 0.82 |
| FLAG-hLHR (exon 9) ([125I]hCG binding) | ND | 0.30 ± 0.22 |
| Receptor . | Intact Cells (ng/106 cells) . | Detergent Extracts (ng/106 cells) . |
|---|---|---|
| Myc-hFSHR ([125I]FSH binding) | 3.2 ± 0.13 | 3.9 ± 0.39 |
| Myc-hLHR ([125I]hCG binding) | 6.3 ± 0.68 | 11.0 ± 0.82 |
| FLAG-hLHR (exon 9) ([125I]hCG binding) | ND | 0.30 ± 0.22 |
Cells were transiently transfected with the indicated constructs and used to measure cell surface (i.e. binding to intact cells) and total [125I]FSH/[125I]hCG binding (i.e. binding to detergent extracts) as described in Materials and Methods. Each value represents the mean ± sem of three or four independent transfections. ND, Not detectable.
The Binding Activity of Receptors Expressing Cell Surface and Total Cells
| Receptor . | Intact Cells (ng/106 cells) . | Detergent Extracts (ng/106 cells) . |
|---|---|---|
| Myc-hFSHR ([125I]FSH binding) | 3.2 ± 0.13 | 3.9 ± 0.39 |
| Myc-hLHR ([125I]hCG binding) | 6.3 ± 0.68 | 11.0 ± 0.82 |
| FLAG-hLHR (exon 9) ([125I]hCG binding) | ND | 0.30 ± 0.22 |
| Receptor . | Intact Cells (ng/106 cells) . | Detergent Extracts (ng/106 cells) . |
|---|---|---|
| Myc-hFSHR ([125I]FSH binding) | 3.2 ± 0.13 | 3.9 ± 0.39 |
| Myc-hLHR ([125I]hCG binding) | 6.3 ± 0.68 | 11.0 ± 0.82 |
| FLAG-hLHR (exon 9) ([125I]hCG binding) | ND | 0.30 ± 0.22 |
Cells were transiently transfected with the indicated constructs and used to measure cell surface (i.e. binding to intact cells) and total [125I]FSH/[125I]hCG binding (i.e. binding to detergent extracts) as described in Materials and Methods. Each value represents the mean ± sem of three or four independent transfections. ND, Not detectable.
Effect of Receptor Complex Formation on [125I]hFSH Binding and cAMP Responsiveness of Transiently Transfected HEK293 Cells
| Receptor . | hFSH Bindinga . | hFSH-Stimulated cAMP Accumulationb . | |||
|---|---|---|---|---|---|
| Kd (nm) . | Binding Capacity (molecules/cell) . | Basal cAMP (pmol/105 cells) . | EC50 (pm) . | Maximal Response (pmol/105 cells) . | |
| Myc-hFSHR | 3.6 ± 0.36 | 38,600 ± 3,500 | 0.8 ± 0.2 | 9.3 ± 1.4 | 86.9 ± 6.4 |
| Myc-FSHR+FLAGhLHR (exon 9) | 3.5 ± 0.34 | 25,300 ± 4,800c | 1.0 ± 0.3 | 4.4 ± 2.2 | 43.2 ± 6.8c |
| Receptor . | hFSH Bindinga . | hFSH-Stimulated cAMP Accumulationb . | |||
|---|---|---|---|---|---|
| Kd (nm) . | Binding Capacity (molecules/cell) . | Basal cAMP (pmol/105 cells) . | EC50 (pm) . | Maximal Response (pmol/105 cells) . | |
| Myc-hFSHR | 3.6 ± 0.36 | 38,600 ± 3,500 | 0.8 ± 0.2 | 9.3 ± 1.4 | 86.9 ± 6.4 |
| Myc-FSHR+FLAGhLHR (exon 9) | 3.5 ± 0.34 | 25,300 ± 4,800c | 1.0 ± 0.3 | 4.4 ± 2.2 | 43.2 ± 6.8c |
HEK293 cells were transfected with either Myc-hFSHR alone or Myc-hFSHR and FLAG-hLHR (exon 9), and cells were used to measure equilibrium binding parameters for [125]hFSH during an overnight incubation at 4 C. Each number represents the mean ± sem of five independent experiments. The binding capacity was significantly lower when Myc-hFSHR was coexpressed with FLAG-hLHR (exon 9) than when expressed alone. (see footnote c)
HEK293 cells were incubated in the presence of 0.5 mm 3-isobutyl-1-methylxanthine with increasing concentrations of hFSH for 30 min at 37 C. The methods used to measure cAMP and to calculate the EC50 and maximal response for hFSH stimulation are described in Materials and Methods. Each value represents the mean ± sem of five independent experiments. Duplicate dishes were used in each experiments. The EC50 was significantly higher and the maximal response was significantly lower when Myc-hFSHR was coexpressed with FLAG-hLHR (exon 9) than when expressed alone. (see footnote c)
P < 0.05.
Effect of Receptor Complex Formation on [125I]hFSH Binding and cAMP Responsiveness of Transiently Transfected HEK293 Cells
| Receptor . | hFSH Bindinga . | hFSH-Stimulated cAMP Accumulationb . | |||
|---|---|---|---|---|---|
| Kd (nm) . | Binding Capacity (molecules/cell) . | Basal cAMP (pmol/105 cells) . | EC50 (pm) . | Maximal Response (pmol/105 cells) . | |
| Myc-hFSHR | 3.6 ± 0.36 | 38,600 ± 3,500 | 0.8 ± 0.2 | 9.3 ± 1.4 | 86.9 ± 6.4 |
| Myc-FSHR+FLAGhLHR (exon 9) | 3.5 ± 0.34 | 25,300 ± 4,800c | 1.0 ± 0.3 | 4.4 ± 2.2 | 43.2 ± 6.8c |
| Receptor . | hFSH Bindinga . | hFSH-Stimulated cAMP Accumulationb . | |||
|---|---|---|---|---|---|
| Kd (nm) . | Binding Capacity (molecules/cell) . | Basal cAMP (pmol/105 cells) . | EC50 (pm) . | Maximal Response (pmol/105 cells) . | |
| Myc-hFSHR | 3.6 ± 0.36 | 38,600 ± 3,500 | 0.8 ± 0.2 | 9.3 ± 1.4 | 86.9 ± 6.4 |
| Myc-FSHR+FLAGhLHR (exon 9) | 3.5 ± 0.34 | 25,300 ± 4,800c | 1.0 ± 0.3 | 4.4 ± 2.2 | 43.2 ± 6.8c |
HEK293 cells were transfected with either Myc-hFSHR alone or Myc-hFSHR and FLAG-hLHR (exon 9), and cells were used to measure equilibrium binding parameters for [125]hFSH during an overnight incubation at 4 C. Each number represents the mean ± sem of five independent experiments. The binding capacity was significantly lower when Myc-hFSHR was coexpressed with FLAG-hLHR (exon 9) than when expressed alone. (see footnote c)
HEK293 cells were incubated in the presence of 0.5 mm 3-isobutyl-1-methylxanthine with increasing concentrations of hFSH for 30 min at 37 C. The methods used to measure cAMP and to calculate the EC50 and maximal response for hFSH stimulation are described in Materials and Methods. Each value represents the mean ± sem of five independent experiments. Duplicate dishes were used in each experiments. The EC50 was significantly higher and the maximal response was significantly lower when Myc-hFSHR was coexpressed with FLAG-hLHR (exon 9) than when expressed alone. (see footnote c)
P < 0.05.
[125I]hFSH Binding and cAMP Responsiveness of Transiently Transfected HEK293 Cells Expressing Different Receptor Levels of Myc-hFSHR
| Receptor . | hFSH Bindinga . | hFSH-Stimulated cAMP Accumulationb . | |||
|---|---|---|---|---|---|
| Kd (nm) . | Binding Capacity (molecules/cell) . | Basal cAMP (pmol/105 cells) . | EC50 (pm) . | Maximal Response (pmol/105 cells) . | |
| Myc-hFSHR (10 μg) | 3.6 ± 0.36 | 38,600 ± 3,500 | 0.8 ± 0.2 | 9.3 ± 1.4 | 86.9 ± 6.4 |
| Myc-hFSHR (5 μg) | 4.1 ± 0.48 | 18,400 ± 2,800c | 1.1 ± 0.5 | 4.2 ± 0.5 | 44.8 ± 7.7c |
| Myc-hFSHR (1 μg) | 5.3 ± 0.62 | 11,300 ± 2,300c | 0.7 ± 0.2 | 7.5 ± 1.0 | 22.5 ± 3.7c |
| Receptor . | hFSH Bindinga . | hFSH-Stimulated cAMP Accumulationb . | |||
|---|---|---|---|---|---|
| Kd (nm) . | Binding Capacity (molecules/cell) . | Basal cAMP (pmol/105 cells) . | EC50 (pm) . | Maximal Response (pmol/105 cells) . | |
| Myc-hFSHR (10 μg) | 3.6 ± 0.36 | 38,600 ± 3,500 | 0.8 ± 0.2 | 9.3 ± 1.4 | 86.9 ± 6.4 |
| Myc-hFSHR (5 μg) | 4.1 ± 0.48 | 18,400 ± 2,800c | 1.1 ± 0.5 | 4.2 ± 0.5 | 44.8 ± 7.7c |
| Myc-hFSHR (1 μg) | 5.3 ± 0.62 | 11,300 ± 2,300c | 0.7 ± 0.2 | 7.5 ± 1.0 | 22.5 ± 3.7c |
HEK293 cells were transfected with different amounts of Myc-hFSHR plasmid, and cells were used to measure equilibrium binding parameters for [125I]hFSH during an overnight incubation at 4 C. Each number represents the mean ± sem of five independent experiments. The binding capacity was significantly lower when Myc-hFSHR was transfected with low amount of plasmid. (see footnote c)
HEK293 cells were incubated in the presence of 0.5 mm 3-isobutyl-1-methylxanthine with increasing concentrations of hFSH for 30 min at 37 C. The methods used to measure cAMP and to calculate the EC50 and maximal response for hFSH stimulation are described in Materials and Methods. Each value represents the mean ± sem of five independent experiment. The maximal response was significantly lower when Myc-hFSHR was expressed with a low amount of plasmid. (see footnote c)
P < 0.05.
[125I]hFSH Binding and cAMP Responsiveness of Transiently Transfected HEK293 Cells Expressing Different Receptor Levels of Myc-hFSHR
| Receptor . | hFSH Bindinga . | hFSH-Stimulated cAMP Accumulationb . | |||
|---|---|---|---|---|---|
| Kd (nm) . | Binding Capacity (molecules/cell) . | Basal cAMP (pmol/105 cells) . | EC50 (pm) . | Maximal Response (pmol/105 cells) . | |
| Myc-hFSHR (10 μg) | 3.6 ± 0.36 | 38,600 ± 3,500 | 0.8 ± 0.2 | 9.3 ± 1.4 | 86.9 ± 6.4 |
| Myc-hFSHR (5 μg) | 4.1 ± 0.48 | 18,400 ± 2,800c | 1.1 ± 0.5 | 4.2 ± 0.5 | 44.8 ± 7.7c |
| Myc-hFSHR (1 μg) | 5.3 ± 0.62 | 11,300 ± 2,300c | 0.7 ± 0.2 | 7.5 ± 1.0 | 22.5 ± 3.7c |
| Receptor . | hFSH Bindinga . | hFSH-Stimulated cAMP Accumulationb . | |||
|---|---|---|---|---|---|
| Kd (nm) . | Binding Capacity (molecules/cell) . | Basal cAMP (pmol/105 cells) . | EC50 (pm) . | Maximal Response (pmol/105 cells) . | |
| Myc-hFSHR (10 μg) | 3.6 ± 0.36 | 38,600 ± 3,500 | 0.8 ± 0.2 | 9.3 ± 1.4 | 86.9 ± 6.4 |
| Myc-hFSHR (5 μg) | 4.1 ± 0.48 | 18,400 ± 2,800c | 1.1 ± 0.5 | 4.2 ± 0.5 | 44.8 ± 7.7c |
| Myc-hFSHR (1 μg) | 5.3 ± 0.62 | 11,300 ± 2,300c | 0.7 ± 0.2 | 7.5 ± 1.0 | 22.5 ± 3.7c |
HEK293 cells were transfected with different amounts of Myc-hFSHR plasmid, and cells were used to measure equilibrium binding parameters for [125I]hFSH during an overnight incubation at 4 C. Each number represents the mean ± sem of five independent experiments. The binding capacity was significantly lower when Myc-hFSHR was transfected with low amount of plasmid. (see footnote c)
HEK293 cells were incubated in the presence of 0.5 mm 3-isobutyl-1-methylxanthine with increasing concentrations of hFSH for 30 min at 37 C. The methods used to measure cAMP and to calculate the EC50 and maximal response for hFSH stimulation are described in Materials and Methods. Each value represents the mean ± sem of five independent experiment. The maximal response was significantly lower when Myc-hFSHR was expressed with a low amount of plasmid. (see footnote c)
P < 0.05.
hLHR(Exon 9) Expresses in Ovary of Normal Women at Every Phase of the Menstrual Cycle
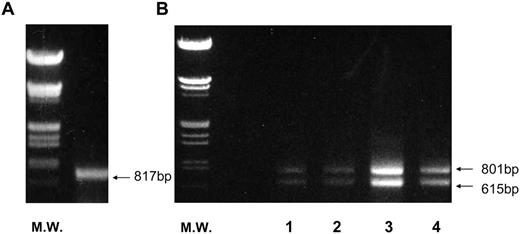
We examined whether hLHR(exon 9) is expressed in the ovary of a normal woman at every phase of the menstrual cycle. We previously showed that the amounts of hLHR mRNA increase from preovulatory follicles to the corpus luteum of midluteal phase and subsequently decrease at the late luteal phase. As shown in Fig. 7B, corresponding to our previous data (7), the expression of both wild-type hLHR (801 bp) and hLHR(exon 9) (615 bp) changed across the luteal phase, and hLHR(exon 9) was seen at every phase of the cycle. To confirm this, a quantitative real-time PCR was performed. The expression at the exon 10–11 region of hLHR indicates the sum expression of both full-length hLHR and hLHR(exon 9). On the other hand, the expression at the exon 9–10 region of hLHR indicates the expression of just the full-length hLHR. The expression of hLHR(exon 9) is the difference between the exon 10–11 region and the exon 9–10 region of hLHR. As shown in Table 4, hLHR(exon 9) was expressed at every phase of the cycle, indicating that hLHR(exon 9) is naturally expressed at all phases of the menstrual cycle. Moreover, the expression of hLHR(exon 9) mRNA relative to hLHR was increased as the luteal phase progressed. Because we detected a single band (817 bp) by RT-PCR with primers to amplify exon 1 to exon 10 (Fig. 7A), we designed one pair of primers for a quantitative RT-PCR. In contrast to hLHR and hLHR(exon 9), FSHR mRNA levels clearly declined from 8000 ± 530 to 3000 ± 320 in copy numbers after ovulation, supporting our previous data (7).
RT-PCR and Quantitative Real-Time PCR of hFSHR and hLHR Transcripts in Human Ovaries Total RNA samples were prepared from the ovary. RT-PCR was performed for hFSHR (l4-d follicle) (A) or hLHR (lane 1, 10-d follicle; lane 2, 14-d follicle; lane 3, 21-d corpus luteum; lane 4, 27-d corpus luteum) (B) as described in Materials and Methods. DNA size markers (M.W.) were provided by digesting λ-phage DNA with BamHI and HindIII. The picture is representative of the three independent experiments.
hFSHR, hLHR, and hLHR (Exon 9) mRNA Levels during the Menstrual Cycle
| . | Menstrual Cycle (d) . | |||
|---|---|---|---|---|
| 10 . | 14 . | 21 . | 27 . | |
| hFSHR | 6,000 ± 480 | 8,000 ± 530 | 3,000 ± 320 | 520 ± 62 |
| hLHR | 5,500 ± 510 | 8,400 ± 790 | 21,000 ± 1,400 | 6,300 ± 570 |
| hLHR (exon 9) | 1,900 ± 400 | 5,000 ± 460 | 22,000 ± 1,600 | 8,600 ± 780 |
| . | Menstrual Cycle (d) . | |||
|---|---|---|---|---|
| 10 . | 14 . | 21 . | 27 . | |
| hFSHR | 6,000 ± 480 | 8,000 ± 530 | 3,000 ± 320 | 520 ± 62 |
| hLHR | 5,500 ± 510 | 8,400 ± 790 | 21,000 ± 1,400 | 6,300 ± 570 |
| hLHR (exon 9) | 1,900 ± 400 | 5,000 ± 460 | 22,000 ± 1,600 | 8,600 ± 780 |
Amounts of hFSHR, hLHR, and hLHR (exon 9) mRNA were determined by quantitative real-time RT-PCR as described in Materials and Methods. Each number represents the means ± sem of three independent experiments. Each mRNA level is shown in copies per 100 ng of RNA.
hFSHR, hLHR, and hLHR (Exon 9) mRNA Levels during the Menstrual Cycle
| . | Menstrual Cycle (d) . | |||
|---|---|---|---|---|
| 10 . | 14 . | 21 . | 27 . | |
| hFSHR | 6,000 ± 480 | 8,000 ± 530 | 3,000 ± 320 | 520 ± 62 |
| hLHR | 5,500 ± 510 | 8,400 ± 790 | 21,000 ± 1,400 | 6,300 ± 570 |
| hLHR (exon 9) | 1,900 ± 400 | 5,000 ± 460 | 22,000 ± 1,600 | 8,600 ± 780 |
| . | Menstrual Cycle (d) . | |||
|---|---|---|---|---|
| 10 . | 14 . | 21 . | 27 . | |
| hFSHR | 6,000 ± 480 | 8,000 ± 530 | 3,000 ± 320 | 520 ± 62 |
| hLHR | 5,500 ± 510 | 8,400 ± 790 | 21,000 ± 1,400 | 6,300 ± 570 |
| hLHR (exon 9) | 1,900 ± 400 | 5,000 ± 460 | 22,000 ± 1,600 | 8,600 ± 780 |
Amounts of hFSHR, hLHR, and hLHR (exon 9) mRNA were determined by quantitative real-time RT-PCR as described in Materials and Methods. Each number represents the means ± sem of three independent experiments. Each mRNA level is shown in copies per 100 ng of RNA.
Cotransfection of FLAG-hLHR(exon 9) Drives Myc-hFSHR to the Lysosome
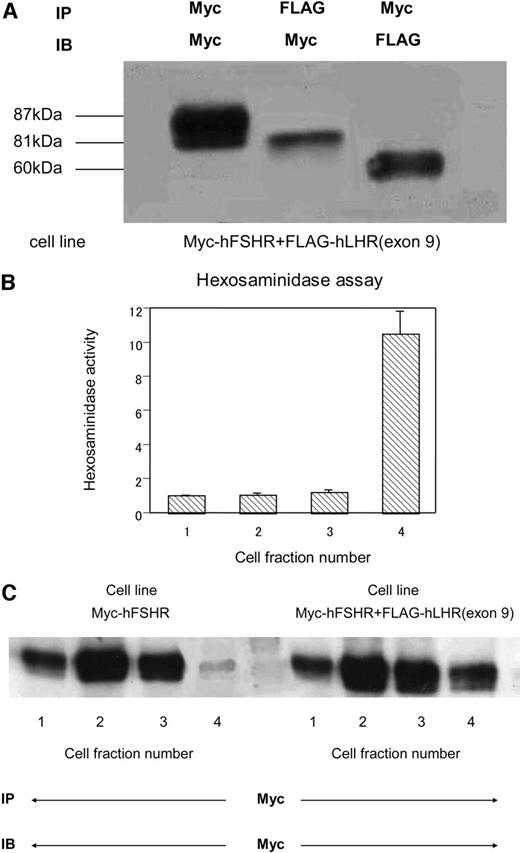
To examine the mechanism of Myc-hFSHR reduction through cotransfection of FLAG-hLHR(exon 9), Percoll gradient was used to fractionate the postnuclear supernatant from cells stably expressing either Myc-hFSHR or FLAG-hLHR(exon 9) or both. At first, we tried to ascertain whether the receptor association between Myc-hFSHR and FLAG-hLHR(exon 9) occurred in stable cell lines. As shown in Fig. 8A, it was clearly demonstrated that the immature receptor of Myc-hFSHR associated with FLAG-hLHR(exon 9), coinciding with data shown in Fig. 3. Next, we measured β-hexosaminidase activity, known as the biochemical marker for the lysosomes, to collect the lysosome fraction. As shown in Fig. 8B, fraction number 4 had a hexosaminidase activity 10 times higher than other fractions, suggesting that the majority of lysosomes are separated into this fraction. The Western blot data in Fig. 8C show that in the cells expressing Myc-hFSHR alone, the 87-kDa band of the mature Myc-hFSHR was prominently depicted in fraction 1, which meant this fraction mainly came from plasma membrane. On the other hand, in lane 4, very faint bands were observed, suggesting that Myc-hFSHR is hardly located in the lysosomal compartment. However, in the cells expressing both Myc-hFSHR and FLAG-hLHR(exon 9), Myc-hFSHR was obviously detected in lane 4. These results suggest that the underlying mechanism of the reduction in Myc-hFSHR expression at the plasma membrane was due to the increase of Myc-hFSHR driven to the lysosome, where the receptors are eventually degraded.
Distribution of Myc-hFSHR and FLAG-hLHR(Exon 9) in Lysosome of HEK293 Cells Stably Expressing Either or Both Myc-hFSHR and FLAG-hLHR(Exon 9) Cells stably express indicated constructs. Receptor numbers are 23,800 ± 4,000 for Myc-hFSHR and 41,800 ± 5,000 for Myc-hFSHR and FLAG-hLHR(exon 9), respectively. The receptor expression level was evaluated by the signal intensity of Western blot. A, HEK293 cells stably expressing both Myc-hFSHR and FLAG-hLHR(exon 9) were solubilized as described in Materials and Methods, immunoprecipitated (IP) with anti-c-Myc-antibody or anti-FLAG-antibody, and resolved by 7.5% reducing SDS-PAGE. After being transferred to a PVDF membrane, confirmed with an indicated antibody (IB). The blot is representative of three independent experiments. B, Postnuclear supernatants of HEK293 cells were prepared and fractionated on Percoll gradients as described in Materials and Methods. The contents of the gradients were then collected from the top (500 μl/fraction), and aliquots of each four fractions were combined to assay β-hexosaminidase activity. The results show the distribution of endogenous β-hexosaminidase activity present in HEK293 cells. Data represent the mean increase relative to the combined fraction number 1. C, HEK293 cells stably expressing either or both Myc-hFSHR and FLAG-hLHR(exon 9) were prepared and fractionated on Percoll gradients as described in Materials and Methods. The contents of the gradients were then collected from the top (500 μl/fraction), and aliquots of each four fractions were combined, immunoprecipitated (IP) with anti-c-Myc-antibody, and resolved by 7.5% reducing SDS-PAGE. After being transferred to a PVDF membrane, confirmed with anti-c-Myc-antibody to detect the expression of Myc-hFSHR. The blot is representative of three independent experiments.
The Association of hFSHR and Different Misfolded hLHR Mutants
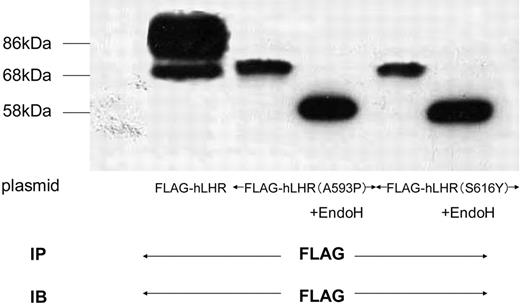
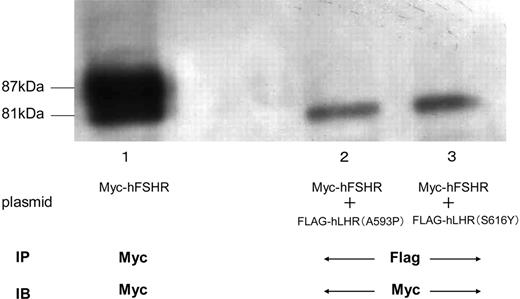
To examine whether Myc-hFSHR forms complexes with different misfolded hLHR mutants, we transiently cotransfected Myc-hFSHR with either of the two different misfolded hLHR mutants. The two mutants, hLHR(A593P) and hLHR(S616Y), are naturally occurring inactivating mutants of the hLHR (14–16). As shown in Fig. 9, these mutants were observed on Western blots as an ER-located misfolded receptor, sensitive to EndoH. In the HEK293 cells coexpressing both Myc-hFSHR and misfolded hLHR mutants, differential immunoprecipitation was used, and immature forms of Myc-hFSHR were coprecipitated with anti-FLAG antibody (Fig. 10 lanes 2 and 3). From these data, the immature form of Myc-hFSHR does indeed interact with FLAG-tagged misfolded hLHR mutants.
Glycosylation Condition of Misfolded hLHR Mutants HEK293 cells expressing either of two different misfolded hLHR mutants (A593P and S616Y), were solubilized in detergent and were incubated in the absence (−) or presence (+) of EndoH as described in Materials and Methods. After incubation, each sample was immunoprecipitated, resolved by 7.5% reducing SDS-PAGE, transferred to a PVDF membrane, and confirmed with M2 anti-FLAG antibody. The blot is representative of three independent experiments. IP, Immunoprecipitation; IB, immunoblot.
Detection of Myc-hFSHR Association with Other Misfolded hLHR Mutants by Immunoblotting and Coimmunoprecipitation HEK293 cells expressing Myc-hFSHR alone (lane 1), or HEK293 cells coexpressing Myc-hFSHR and FLAG-hLHR(A593P) (lane 2), or HEK293 cells coexpressing Myc-hFSHR and FLAG-hLHR(S616Y) (lane 3) were solubilized as described in Materials and Methods, immunoprecipitated (IP) with anti-c-Myc antibody (lane 1) or anti-FLAG-antibody (lanes 2 and 3), and resolved by 7.5% reducing SDS-PAGE. After being transferred to a PVDF membrane, confirmed with anti-c-Myc-antibody (IB). The blot is representative of three independent experiments.
DISCUSSION
In our study, Western blot and coimmunoprecipitation experiments clearly demonstrate that Myc-hFSHR and FLAG-hLHR(exon 9) form a receptor complex, which abolished the function of hFSHR in terms of cAMP production, resulting in the reduction of Myc-hFSHR expression at the cell surface. Furthermore, the expression of FLAG-hLHR(exon 9) induced the association with Myc-hFSHR, which, in turn, reduced the immunoreactive receptor protein, confirmed by Western blot. Consequently, this association attenuated cAMP production for signal transduction.
Classical models of GPCRs had been thought to function as a monomer, which may be oversimplified because an increasing number of reports have demonstrated that many GPCRs exist as dimers or oligomers. GPCRs, either from the same or different families, were found to form heterodimers. In most cases, heterodimers were demonstrated by coimmunoprecipitation and examined for functional studies including agonist-induced signal transduction, desensitization, and endocytosis. Because a more definitive determination of the stoichiometry of each complex is absent, we could not use the term “dimerization” between Myc-hFSHR and FLAG-hLHR(exon 9). However, the coimmunoprecipitation study in Fig. 3 clearly shows that Myc-hFSHRs form an association with FLAG-hLHR(exon 9) in the HEK293 cells, followed by a reduction of the Myc-hFSHR expression at the cell surface. There are several published reports describing the heterodimerization of distinct GPCRs, e.g. the heterodimeric of δ-opioid receptor and β2AR-influenced internalization (17). In the case of type 2A somatostatin receptor (SSTR2A)-μ opioid receptor heterodimerization, the receptor cross-phosphorylation and cross-desensitization were evoked (18); those were not observed with SSTR2A-SSTR3 heterodimer. In our case, FLAG-hLHR(exon 9) formed a receptor complex with the 81-kDa immature band of Myc-hFSHR, resulting in the attenuation of the protein level of Myc-hFSHR (Fig. 5). As shown in Figs. 1 and 2, FLAG-hLHR(exon 9) was demonstrated to be retained in the ER, where FLAG-hLHR(exon 9) could form a receptor complex with the immature receptor of Myc-FSHR. This suggested that the receptor complex was assembled during biosynthesis in the ER. Furthermore, as shown in Fig. 10, we detected that Myc-hFSHR could form a receptor complex with FLAG-hLHR(A539P) or FLAG-hLHR(S616Y). Tao et al. (19) reported that the ER-localized misfolded hLHR mutant could associate with the immature hLHR. Taken together with our previous report (20) describing the association of hLHR(exon 9) with the immature hLHR in the ER, we propose that the misfolded mutant hLHR forms a receptor association with wild-type gonadotropin receptors. This phenomenon cannot occur with TSHR, categorized in the same subclass of GPCR with the large glycosylated extracellular domain (Fig. 4). However, processes involved in the formation of the receptor complex remain poorly characterized.
To elucidate the mechanism underlying the reduction of Myc-hFSHR protein level by the coexpression with FLAG-hLHR(exon 9), we adapted the Percoll gradient fractionation. As shown in Fig. 8C, the expression of FLAG-hLHR(exon 9) formed the association with Myc-hFSHR, where this receptor complex is driven to the lysosomal fraction and eventually degraded. Human chemokine receptor 5 (CCR5) belongs to GPCR, which also has a natural deletion mutant (an internal 32-nucleotide deletion), CCR5▵32. Likewise, FLAG-hLHR, CCR5▵32 remains in the ER to reduce cell surface expression of CCR5(21), perhaps in the same way as FLAG-hLHR(exon 9) reduced the expression of Myc-hFSHR at the plasma membrane. However, the mechanism for this reduction is not yet explored. In the case of LHR, either naturally occurring or experimentally designed mutant LHRs have been described to result in extremely reduced levels of cell surface expression (22, 23). In most cases, whether decreased cell surface expression is due to increased degradation of the mutant receptor and/or increased intracellular retention has also not yet been explored. Some molecules, such as a chaperone, may participate in the control mechanism, facilitating the recognition of the mutant receptor as inappropriate and transporting it to the lysosome instead of the cell surface. Because chaperone protein calnexin has been demonstrated to interact with FSHR and LHR (24), we speculate that Myc-hFSHR, in association with FLAG-hLHR(exon 9), disables calnexin to recognize Myc-hFSHR as a properly folded receptor, which is then led to the degradation pathway toward the lysosome.
In the RT-PCR experiment (Fig. 7), we detected hLHR(exon 9) mRNA from the late follicular phase to the late luteal phase. Therefore, both hLHR and hLHR(exon 9) are expressed during the entire luteal phase. To quantify the mRNA levels of hFSHR, hLHR, and hLHR(exon 9), we performed RT-PCR (Table.4). As the luteal phase progressed, hLHR(exon 9) increased relative to hLHR, demonstrating that hLHR(exon 9) expressed more than hLHR in the late luteal phase. On the other hand, hFSHR mRNA levels were markedly decreased after ovulation, consistent with the previous results from this laboratory (25). These results suggested that hLHR(exon 9) dominantly existed against hFSHR from the postovulation to the early luteal phase. Given that hFSHR forms the receptor complex with hLHR(exon 9) in the physiological condition, this association could negatively regulate hFSHR expression as hLHR(exon 9) is increasingly expressed.
Recently, many studies were conducted to analyze the functional modulation of receptor dimer or oligomer formation. They have provided us some insight in understanding the functional properties including ligand binding, signaling, and receptor trafficking. Signal transduction inside the cell starts with the interaction between the receptor and the G protein, which raises the possibility that the attenuation of FSHR signal transduction mediated by cAMP was due to the consumption of G protein reservoir in the cytosol. However, we do not think that is the case for hFSHR and hLHR(exon 9) receptor complex because hLHR(exon 9) remains in the ER where the receptor cannot interact with G protein. Thus, from the results as shown in Tables 2 and 3, we concluded that the attenuation of FSHR signal transduction mediated by cAMP was due to the reduction in receptor number at the cell surface without changing the binding affinity.
hLHR(exon 9) codes leucine-rich repeats 8–9 and N-terminal part of hinge region. There are many reports conducting the functional analysis of the N-terminal extracellular domain. The deletion of exon 10 of the hLHR associated with Leydig cell hypoplasia (6) shows a reduction in hCG binding at the cell surface in transfected cells, but not inside the cells, assessed by detergent extract binding assay (26). On the other hand, the mutant hLHR lacking exon 8 demonstrates the loss of both cell surface and total cell binding to hCG (5). In another line of experiments, deletions of leucine-rich repeats 1–6 of rat LHR coding exon 1–7 cause a loss of binding ability to hCG, and deletions of leucine-rich repeats 9–14 coding exon 8–10 cause a trap of the receptor intracellularly in a form capable of binding hCG (27). Through our receptor biotinylation experiment, however, we clearly showed that FLAG-hLHR(exon 9) is not expressed on the cell surface (Fig. 2). Based on the comparison between the effects of EndoH and N-GlycoF digestion of Myc-hFSHR and FLAG-hLHR(exon 9), as shown in Fig. 1, FLAG-hLHR(exon 9) is shown to be retained in the endoplasmic reticulum. Moreover, FLAG-hLHR(exon 9) cannot bind to hCG, proven by the binding experiment with a nonionic detergent and glycerol (Table 1) in which the precursor of the wild-type LHR can bind hCG with the same affinity as cell surface receptor (28). Studies addressing the role of exons 8–10 have, however, provided conflicting views of their function. As mentioned above, some reports have suggested that exons 8–10 of LHR are not required for hormone binding, whereas others have suggested a requirement for these regions with respect to hormone binding and/or correct folding of the nascent LHR. Although most investigators argue that exons 9 and 10 are not necessary for binding (22), our data raised the possibility that exon 9 was involved in binding. Zeng et al. (29) have reported that the hinge region of the rat LHR is involved in hormone interaction and signal interaction. There is an another study that suggested Ser-277, which exists within exon 9 in the hinge region, causes conformational change, resulting in constitutive receptor activation (30). In addition, cysteine residues 257 and 258 encoded in exon 9 of rat LHR, conserved in all of the glycoprotein hormone receptors, resulted in total loss of surface binding activity, whereas the hormone binding activity was recovered as much as 70–100% of wild-type rat LHR in detergent (31). In many cases, only one to five amino acid differences have been shown to dramatically change receptor function including receptor trafficking, hormone binding, and signal transduction. Taken together, we hypothesize that exon 9 of hLHR is involved in both receptor insertion to the plasma membrane and hormone binding, based on the fact that exon 9 codes leucine-rich repeats 8–9 and N-terminal part of hinge region. Whether this hypothesis turns out to be true in the case of hLHR will require further study.
Due to the limitation of collecting samples from the human ovary, definitive evidence about the regulation of hFSHR expression in vivo has not yet been reported. Thus, animal models, especially rat, have been used for those types of studies. Previously, we used immature rats treated with PMSG to stimulate follicular growth resulting in an increase of FSHR expression, whereas subsequent hCG administration to induce ovulation and luteinization dramatically decreased FSHR expression. Meanwhile, LHR expression began to increase according to luteinization after hCG administration. Based on the fact that FLAG-hLHR(exon 9) can antagonize Myc-hLHR expression, this study would add a new model with which to examine the underlying mechanism of FSHR expression regulation during the menstrual cycle.
MATERIALS AND METHODS
Hormones and Reagents
Purified hFSH (NIADDK-hFSH-I-3) was provided by the National Hormone and Pituitary Program (NIDDK, Baltimore, MD). This material was used for iodinations and in experiments designed to measure intracellular cAMP levels. Anti-cAMP serum was donated by Dr. Takashi Matozaki (Biosignal Research Institute for Molecular and Cellular Regulation, Gunma University, Japan). [125I]Sodium was purchased from Amersham Pharmacia Biotech (Arlington Heights, IL).
Construction of hFSHR, hLHR, hLHR(exon 9), and hTSHR Expression Vectors
cDNAs encoding hFSHR, hLHR, hLHR(exon 9), and hTSHR were inserted separately into the mammalian expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA). The receptors were tagged with c-Myc for wild-type hFSHR (Myc-hFSHR) or FLAG epitopes for wild-type hLHR(FLAG-hLHR), hLHR(exon 9) [FLAG-hLHR(exon 9)], hLHR(A593P) [FLAG-hLHR(A593P)], hLHR(S616Y) [FLAG-hLHR(S616Y)], and hTSHR (FLAG-hTSHR). The receptor cDNAs were modified using PCR to insert, after signal peptides, a 10-residue c-Myc epitope (EQKLISEEDL) for the Myc-hFSHR and an eight-residue FLAG epitope (DYKDDDDK) for the FLAG-hLHR, FLAG-hLHR(exon 9) and FLAG-hTSHR. Their identities were verified by automated DNA sequencing on both strands.
Expression in Mammalian Cells
HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in DMEM containing 10 mm HEPES, 10% newborn calf serum, and 50 μg/ml gentamicin, pH 7.4. Transient transfections were done using the calcium phosphate method of Chen and Okayama (32). Cells were plated in 100-mm cell culture dishes and transfected with 10 μg of plasmid DNA for a single transfection or 20 μg plasmid DNA for cotransfection when 70–80% confluent. For each experiment, the transfected HEK293 cells were used 2 d after transfection. To establish cell lines stably, HEK293 cells were plated into 100-mm dishes and transfected with either Myc-hFSHR or FLAG-hLHR(exon 9) or with both constructs. Cells were selected in media containing 700 μg/ml G418. Stable cell lines were maintained in the media containing G418.
SDS-PAGE, Western Blotting, and Immunoprecipitation
HEK293 cells expressing receptors were lysed in lysis buffer [0.5% Nonidet P-40, 200 mm NaCl, 20 mm HEPES, 1 mm EDTA (pH 7.4) containing protease inhibitors] during a 30-min incubation at 4 C.
The lysates were clarified by centrifugation (100,000 × g for 30 min), and the amount of protein present in supernatant of the lysates was measured using the DC protein assay (Bio-Rad Laboratories, Inc., Hercules, CA). For immunoprecipitation, the lysates were incubated with the agarose-conjugated anti-c-Myc antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or the agarose-conjugated anti-FLAG M2 antibody (Sigma Chemical Co., St. Louis, MO) at 4 C overnight.
Immunocomplexes were eluted by incubation in sodium dodecyl sulfate sample buffer for 1 h at room temperature. The eluant was then resolved on sodium dodecyl sulfate gels and electrophoretically transferred to polyvinyldenefluoride (PVDF) membranes as described elsewhere (33). After blocking, expression of the different protein was determined with the HRP-conjugated anti-c-Myc antibody (Santa Cruz) or the HRP-conjugated anti-FLAG M2 antibody (Sigma). The proteins were finally visualized using ECL-Plus (Amersham).
EndoH and N-GlycoF Treatment of Myc-hFSHR and FLAG-hLHR(Exon 9)
Detergent-soluble extracts of HEK293 cells expressing Myc-hFSHR or FLAG-hLHR(exon 9) were incubated in the presence of EndoH or N-GlycoF (Roche Diagnostics Corp., Indianapolis, IN). EndoH treatment was performed by incubation of detergent-soluble extracts with 300 mU/ml EndoH for 15 h at 37 C, and N-GlycoF treatment was done with 32 U/ml N-GlycoF for 15 h at 37 C. After treatment with each enzyme, detergent-soluble extracts were further treated, followed by immunoprecipitation, SDS-PAGE, and Western blotting as described in SDS-PAGE, Western Blotting, and Immunoprecipitation.
Biotinylation of Receptor
Transfected cells were washed four times with ice-cold PBS (10 mm sodium phosphate, 150 mm NaCl, pH 8) and then biotinylated during a 30-min incubation with freshly prepared 0.5 mg/ml solutions of sulfosuccinimidyl-6-(biotinamid) hexanoate (Pierce Chemical Co., Rockford, IL) in PBS. The cells were lysed and immunoprecipitated for Western blotting as described in SDS-PAGE, Western Blotting, and Immunoprecipitation except that HRP-conjugated streptavidin (Pierce) was used to detect biotinylated receptors.
Binding Assays
To measure equilibrium binding parameters of intact cells, cells were washed and placed in 1 ml of assay medium (Waymouths MB752/1 without NaHCO3, but containing 20 mm HEPES, 50 μg/ml of gentamicin and 1 mg/ml BSA, pH 7.4). After cooling they were incubated with increasing concentrations of [125I]hFSH overnight at 4 C. At the end of the overnight incubation, the cells were scraped from the wells, and they were collected and washed twice using cold wash medium (Hanks’ balanced salt solution modified to contain 1 mg/ml BSA) by centrifugation. Cell pellets were counted directly in a γ-counter. Detergent extracts used to measure [125I]hFSH binding of whole cells were obtained by solubilizing the cells in 0.5% of Nonidet P-40, 20 mm HEPES, 100 mm NaCl, 20% glycerol, and 1 mm EDTA, (pH 7.4), using a constant ratio of 100 μl of detergent solution /1 million cells. Soluble extracts were incubated with [125I]hFSH in the same manner as intact cells. Three wells and triplicate aliquots of the extracts were used for each concentration of [125I]hFSH; two of them received [125I]hFSH only, but the third one also received 50 IU/ml crude hFSH and was used to correct for nonspecific binding. The free and bound hormones were separated as described elsewhere (34).
cAMP Measurement
To obtain concentration-response curves for the hFSH-induced increases in cAMP, the double-antibody RIA method was used to measure intracellular cAMP levels in cells (plated in 35-mm wells) that had been incubated with seven different concentrations of hFSH for 30 min at 37 C in 1 ml of Waymouth MB 752/1 medium containing 50 μg/ml gentamicin, 20 mm HEPES, and 1 mg/ml BSA in the presence of a phosphodiestrase inhibitor, 0.5 mm 3-isobutyl-1-methylxanthine (Sigma). The different parameters that describe the concentration-response curves were calculated as described elsewhere (35).
Tissue Preparation
Human ovaries were removed from patients who had undergone salpingoophorectomy for gynecological diseases. Pathological examination of the ovaries revealed no apparent abnormalities, and none of the patients had received hormone treatment for 6 months before surgery. Informed consent was obtained from all human subjects, and this study was approved by the Gunma University School Institutional Review Board. The menstrual history, basal body temperature record, and/or endometrial histology of the patients were used to determine the date of menstrual cycle.
RNA Extraction, RT-PCR, and Quantitative RT-PCR
After whole ovaries were removed, leading follicles (10 d and 14 d follicle) and corpora lutea (21 d and 27 d corpus luteum) were isolated and stored in liquid nitrogen until use. For PCR amplification, 5 μg of RNA was used to generate first-strand cDNA using a cDNA synthesis kit (SuperScript First-Strand Synthesis System for RT-PCR, Invitrogen) following the manufacturer’s instructions.
The entire 2-μl cDNA synthesis reaction volume was combined in a 100-μl final reaction volume for PCR amplification containing 0.25 μm of each oligonucleotide primer and 1.5 IU Taq DNA polymerase (Sigma). The primer sequences were:
5′-TTCGGATCC-TACATCTGGAGAAGATGCACAATG(LHR-S1) 3′-TCGAGAATTC-AGGTGAATAGCATAGGTGATGGTG(LHR-AS2) for hLHR
5′-CTCACCAAGCTTCGAGTCATCCAA(hFSHR-exon1-F)
3′-TCAAATCCTCTGCTGTAGCTGGAC(hFSHR-exon10-R) for hFSHR, and
5′-CCAAGGTCATCCATGACAACT(GAPDH-F)
3′-CACCCTGTTGCTGTAGCCAAA(GAPDH-R) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
In all, 30 cycles of PCR amplification were performed using a DNA thermal cycler. Each cycle consisted of 90 sec denaturation at 95 C, 150 sec annealing at 62 C, and 150 sec at 70 C for enzymatic extension. After DNA amplification of the PCR mixture, amplified DNA fragments were recovered from agarose gels by the QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA). After precipitation with ethanol, DNA fragments were subcloned into pGEM-T Easy Vector Systems (Promega Corp., Madison, WI). Real-time quantitative PCR was carried out using the ABI Sequence Detection System (Applied Biosystems, Foster City, CA). QuantiTect SYBR Green PCR Master Mix (QIAGEN) was used for all reactions in the ABI Sequence Detection System, and conditions were optimized for the real-time PCR as recommended by the manufacturer. The primers sequences were:
5′-ACACTTTATTCTTCCATGCTTGCTGAG(hLHRexon10–11F) 3′-ATTAAAAGCATCTGGTTCAGGAGCACA(hLHRexon10–11R) to amplify the region between exon 10 and exon 11 of hLHR, 5′-TGCTGTGCTTTTAGAAACTTGCCAACA(hLHRexon9–10F)
3′-CCTTACTGTGCTTTCACATTGTTTGGA(hLHRexon9–10R) to amplify the region between exon 9 and exon 10 of hLHR, and 5′-CTCACCAAGCTTCGAGTCATCCAA(hFSHR-exon1-F), 3′-AAGGTTGGAGAACACTCTGCCTCT(hFSHR-exon3-R) to amplify the region between exon 1 and exon 3 of hFSHR. The assay analysis was carried out using the ABI PRISM 7000 SDS-software.
Cell Fractionation
The cells were washed twice with cold homogenization buffer (0.25 m sucrose, 10 mm HEPES, 1 mm EDTA, pH 7.4), scraped into a small volume of the same buffer, and collected by centrifugation at 4 C. The cells were then resuspended in cold homogenization buffer, and they were lysed by forcing them through a 21-gauge needle 10 times. Postnuclear supernatants were prepared by centrifugation of the homogenates at 800 × g for 10 min at 4 C. The supernatants were saved, and the pellets were rehomogenized and centrifuged again. The two supernatants were combined, and a 2.0-ml aliquot was thoroughly mixed with 6.0 ml of a Percoll solution (with a density of 1.046 g/ml) prepared in homogenization buffer. These mixtures were then centrifuged at 33,000 × g for 20 min at 4 C. The contents of the gradients were then collected from the top (500 μl/fraction), and aliquots of each fraction were further treated, followed by immunoprecipitation, SDS-PAGE, and Western blotting as described in SDS-PAGE, Western Blotting, and Immunoprecipitation.
Other Methods
Statistical analysis (t test with two-sided P values) was performed using StatFlex (Artech, Inc., Osaka, Japan).
Acknowledgments
We thank Dr. Yumiko Abe and Hiroko Matuda for their expert technical assistance. We also thank Dr. Takashi Matozaki (Biological Research Center Institute for Molecular and Cellular Regulation, Gunma University, Gunma, Japan) for anti-cAMP serum.
This work was supported by Uehara Memorial Foundation (Japan), Kanzawa Medical Foundation (Japan), and a grant-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations:
- β-AR,
β-Adrenergic receptor;
- CCR5,
human chemokine receptor 5;
- CG,
choriogonadotropin;
- EndoH,
endoglycosidase H;
- ER,
endoplasmic reticulum;
- FSHR,
FSH receptor;
- GAPDH,
glyceraldehyde-3-phosphate dehydrogenase;
- GPCR,
G protein-coupled receptor;
- HEK,
human embryonic kidney;
- HRP,
horseradish peroxidase;
- LHR,
LH receptor;
- N-GlycoF,
N-glycosidase F;
- PVDF,
polyvinyldenefluoride;
- SSTR,
somatostatin receptor;
- TSHR,
TSH receptor.