Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) binds and activates the aryl hydrocarbon receptor (Ah-R), an endogenous transcription factor that is expressed in the thymus. TCDD exposure leads, among other effects, to thymus atrophy and immunosuppression. We previously analyzed the interference of TCDD with differentiation processes in fetal thymus organ cultures and found that in the presence of TCDD, the proliferation rate of immature (CD4−CD8− and CD4−CD8+ HSA+) thymocytes is inhibited, whereas the maturation along the CD4/CD8 path is accelerated. Moreover, the differentiation of thymocytes is skewed by TCDD at ≤40% (compared with ∼15% without TCDD) of the CD8 single-positive subset of future cytotoxic T cells, and apparently more cells audition for and pass positive selection. The fetal murine thymus expresses functional Ah-R mRNA, as shown by reverse transcription-polymerase chain reaction and TCDD-inducible CYP1A1 and CYP1B1 expression. Because the differentiation of thymocytes is to a considerable extent controlled by cytokines and many cytokine genes are potential targets of the Ah-R due to Ah-R-binding elements (xenobiotic response elements) in their promoters, we analyzed the cytokine expression in fetal thymus organ culture exposed to TCDD. Fetal thymi were cultured from gestation day 15 for ≤8 days, thus coveringex vivo the period after population of the thymus anlage until birth. We show with semiquantitative reverse transcription-polymerase chain reaction that more interleukin (IL)-1β, IL-2, IL-6, tumor growth factor (TGF)-β3, and tumor necrosis factor-α are produced in TCDD-exposed thymi, whereas other cytokines (e.g., TGF-β1, PAI-2, or IL-4) are only slightly up- and down-modulated during the culture period or not modulated at all (e.g., IL-1β, IL-7, interferon-γ, and TGF-β2).
The thymus is the organ responsible for the generation of functional T cells. Intrathymic stem cells from the yolk sac and fetal liver colonize the murine thymus anlage on day 10 or 11 of gestation (1). They differentiate within the thymus in a series of at first synchronized differentiation and selection events, which lead to the generation of an immunologically competent T-lymphocyte repertoire (2). Eventually, in the neonate and adult thymus, a steady state is reached of stem cells entering the thymus, differentiating to maturity, and emigrating to the periphery (3). The developmental processes are coordinated and controlled either intrinsically (notably for the rearrangement of the TCR genes) or externally by interaction of TCR molecules with peptides presented on the MHC molecules of thymic epithelial cells and by cytokines (2). The role of the cytokines is not completely understood. They can be produced by the diverse stromal cell types and by thymocytes themselves (4-6). Studies in which cytokines were added in vitro or constitutively expressed by transgenic mice have suggested cytokines (e.g., IL-4, TNF-α, IL-6, IL-7, IL-10, TGF-β, and IFN-γ) are involved in intrathymic development (7, 8). However, only IL-7 was found to be mandatory for thymocyte development in mice made deficient for cytokines by gene disruption (9).
TCDD, an ubiquitous environmental pollutant, apparently exerts its diverse toxic effects, such as interference with fatty acid metabolism or immunosuppression, by inadvertently activating the endogenous Ah-R (10-12). The Ah-R is a transcription factor abundant in thymus, lung, and liver. It may induce expression of an entire battery of genes (13) by virtue of so-called xenobiotic response elements in their promoters. Hallmarks of low-dose TCDD exposure in experimental animals are induction of the detoxification enzyme CYP450 1A1, notably in the liver; atrophy of the thymus; and, at higher doses, atrophy of other lymphoid organs (14, 15). For many genes, including the cytokine genes IL-1, IL-2, IL-6, TGF-β1, and others (16), it has been shown that their promoters contain DREs, making them putative targets for the TCDD/Ah-R complex (16). With respect to the TCDD-related processes in the thymus, we previously demonstrated in mice that TCDD has a profound effect on the differentiation of thymocytes, such as inhibition of proliferation, changes in the differentiation kinetics, a higher frequency of cells auditioning for positive selection, and increased emigration rates of certain thymocyte subsets. Eventually, the thymus is atrophied, and mature thymocytes are skewed toward CD4−CD8+ single-positive cells (17-21). Recently, an Ah-R-deficient strain of mice was generated by genetic disruption, and these mice displayed hepatic fibrosis and an impaired lymphoid development, again demonstrating the importance of this transcription factor for the immune system (22). Because cytokines may be involved in thymocyte differentiation, we investigated whether TCDD changes cytokine production patterns in the developing thymus, thus causing the diverse effects of TCDD on thymocytes. We provide data from RT-PCRs of thymocytes of fetal thymi kept in organ culture for ≤8 days in the presence or absence of TCDD and show that TCDD indeed modulates the expression kinetics of several cytokines relevant for thymus development.
Materials and Methods
Animals.
Male and female C57BL/6 mice were purchased from the Zentralinstitut für Versuchstierkunde (Hannover, Germany) and kept in our animal facility under standard conditions. Mice were mated overnight, and the following day was counted as day 0 of gestation. Pregnant dams were killed on day 15 of gestation by CO2asphyxiation, and fetuses were removed immediately.
FTOC.
Six randomly chosen thymus lobes that had been collected aseptically from 15-day-old C57BL/6 embryos and freed of adhering tissue were placed on a nitrocellulose filter insert (pore size, 45 μm; Millipore, Bedford, MA) that was set in a 24-well culture plate with 300 μl of culture medium (RPMI 1640 supplemented with 5% fetal calf serum, 5 × 10−4 mβ-mercaptoethanol, 100 units/ml penicillin, 0.1 mg/ml streptomycin). TCDD (purity, >99%; Ökometric GmbH, Bayreuth, Germany) dissolved in 1,4-dioxane (Merck, Darmstadt, Germany) was added to a final concentration of 1 or 10 nm in 0.1% solvent. Control lobes were cultivated in medium plus solvent. No effect of 1,4-dioxane on FTOCs compared with thymi cultivated in medium alone was detectable (data not shown). The thymus lobes were incubated at 37° and 6% CO2 in a water-saturated atmosphere. Cultures were fed on day 4 by transferring the filter inserts to a new well with fresh medium with or without TCDD or solvent. At the indicated days, single-cell suspensions were prepared by gently homogenizing the thymus lobes in phosphate-buffered saline in a small plastic homogenizer. Viability of cells was always >95% as judged by trypan blue exclusion. The cell suspensions from two to four independent experiments were stored at −80° and pooled for RNA preparation.
RNA preparation.
Total RNA was extracted from the frozen thymus preparations using TRIzol reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer’s instruction. The final RNA concentrations were determined spectrophotometrically. Isolated RNA was treated with DNase I Amplification Grade (Life Technologies) at 1 unit of DNase I/μg of RNA in 20 mmTris·HCl, 50 mm KCl, and 2 mmMgCl2 (15 min, RT). The reaction was stopped with 2.0 mm EDTA for 10 min at 60°.
RT reactions.
For the cDNA synthesis reactions, 1 μg of total RNA was preincubated in 10 μl with 1 μg of oligo pd(T)16 primer (Pharmacia, Uppsala, Sweden) for 5 min at 60°. RNA was reverse transcribed in a final volume of 40 μl containing 20 units of RNasin Ribonuclease Inhibitor (Promega, Madison, WI), 400 units of Moloney murine leukemia virus reverse transcriptase (Life Technologies), 1× RT buffer (50 mm Tris·HCl, 75 mm KCl, 3 mm MgCl2), 10 mm dithiothreitol, and 1 mm concentration of each deoxynucleotide triphosphate (i.e., dATP, dCTP, dGTP, dTTP) (Pharmacia, Piscataway, NJ). The RT reactions were carried out for 60 min at 37°, and the samples were then heated to 70° for 10 min. Samples were cooled to 4° and stored at −80° until use.
PCRs.
PCRs were performed in 50 μl of reaction volume containing 5 μl of cDNA (see above), 2.5 units of Taq DNA polymerase (Promega), 1× PCR buffer (50 mm KCl, 10 mm Tris·HCl, 0.1% Triton X-100, and 2.5 mmMgCl2), 0.2 mm concentration of each dNTP containing 1 μCi of [α-32P]dCTP (ICN, Irvine, CA), and 0.25 μm concentration of each primer. Amplifications were carried out on a Biometra Trio-Thermoblock (Biometra Inc., Tampa, FL) for 5 min at 94° before the first cycle, 1 min for denaturation at 94°, 1 min 30 sec for primer annealing, 1 min for extension at 72°, and 10 min at 72° after the last cycle. The annealing temperature and cycles for different cDNAs are shown in Table1.
Oligonucleotide primers used for gene expression analysis by RT-PCR
HPRT, a house-keeping gene, was amplified together with the cDNA of interest in the same reaction tube. To analyze the amplification of both cDNAs in the linear range, the “primer-dropping” method (23) was used to calibrate for the different expression levels of HPRT versus cytokine cDNAs. Briefly, the cDNAs to be tested were first amplified for several cycles (depending on their previously established abundance), and then HPRTprimers were dropped into the reaction tubes followed by an additional 25 cycles.
After PCR amplification, 40 μl of radioactive PCR products were separated on 10% polyacrylamide gels. The gels were dried and autoradiographed.
PCR primers.
Primer pairs of mouse IL-1β, IL-2, IL-4, IL-6, IL-7, TGF-β1, TNF-α, and IFN-γ were obtained from Clontech Laboratories (Palo Alto, CA). Mouse CYP1A1, CYP1B1, Ah-R, and ARNT primers were a kind gift from Drs. Josef Abel and Christoph Vogel (Düsseldorf, Germany). Primer sequences for mouse IL-1β, TGF-β2, TGF-β3, and PAI-2 were chosen from the GenBank sequences with the Oligo 4.0 program (National Biosciences, Plymouth, MN). Primer sequences were checked against the GenBank using the FASTA program. The differences in melting temperatures between the 5′ and 3′ primers were <0.2–3.2°. PCR primers were synthesized with an Applied Biosystems 391 DNA synthesizer (Weiterstadt, Germany) and purified by NAP 5 columns (Pharmacia, Uppsala, Sweden). Table 1 shows the sequences of primers and the size of PCR products by RT-PCR. There was almost no dimer formation with these primers.
Data analysis.
The autoradiographs were scanned and analyzed with an OmniMedia scanner and the Bio Image program (Millipore, Ann Arbor, MI). Quantifications were done on autoradiographs in the linear range of radioactivity to photosensitivity. The relative mRNA levels were calculated as follows: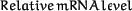
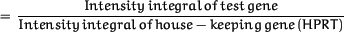 The cytokine gene expression in TCDD-treated fetal thymus lobes is expressed as percentage of untreated control lobes:
The cytokine gene expression in TCDD-treated fetal thymus lobes is expressed as percentage of untreated control lobes:
Restriction endonuclease mapping and Southern blot analysis of PCR products.
Amplified IL-1α and IL-2 PCR products were purified by QIAquick spin column (Qiagen GmbH, Hilden, Germany) and digested with XbaI or HindIII (Life Technologies) followed by separation on a 2% agarose gel and determination of fragment length.
For Southern blot analysis of IL-2, PCR products were detected with the DIG Luminescent Detection Kit (Boehringer-Mannheim Biochemica, Mannheim, Germany) according to the instructions of the manufacturer and using an internal IL-2 oligoprobe (5′-TGC GCT GTT GAC AAG GAG CAC AAG TGT-3′).
Sequence analysis.
Amplified ARNT PCR products were cloned into a T vector prepared by using pBluescript II KS (±) phagemid (Stratagene, La Jolla, CA). Then, 1.5 μg of phagemid DNA was sequenced with the T7 Sequencing Kit (Pharmacia) according to the manufacturer’s instruction and using the RT-PCR primers for ARNT.
Results
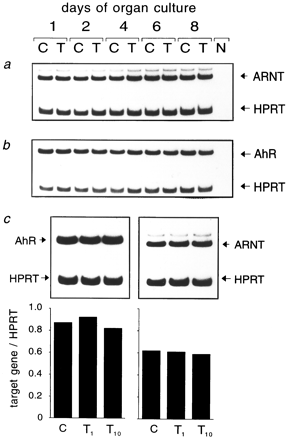
TCDD does not alter Ah-R and ARNT mRNA expression in FTOC.
TCDD mediates most of its biological actions as a part of the transcription factor complex of TCDD and the Ah-R/ARNT heterodimer. The adult thymus of mice has been shown to contain the Ah-R in abundance (24, 25), and the presence of ARNT has been demonstrated in rat thymus (25). owever, little is known about the expression levels and inducibility of Ah-R and ARNT in the fetal thymus. We therefore determined the levels of mRNA expression of the Ah-R and ARNT in FTOC, which would be a prerequisite of TCDD effects. Moreover, we tested whether these two genes are inducible by TCDD. The results are shown in Fig. 1. Both Ah-R and ARNT are easily detectable at the first day of organ culture, which corresponds to day 16 of gestation. The expression was lower than that of HPRT, as evident by having had to drop in the HPRT primers five cycles before starting the Ah-R or ARNT amplification. However, for some cytokine genes, to detect the cDNA, 10 or 15 PCR cycles (see Table 2) had to be run before the addition of the HPRT primers. Densitometric scanning verified that the overall expression levels of Ah-R or ARNT mRNA did not change during ontogenic development, nor was either expression modulated by the presence or absence of even high TCDD concentrations during cultivation.
FTOCs of 15-day-old embryos were treated with TCDD (T) or solvent alone (C). mRNA was prepared, reverse transcribed, and amplified by PCR for Ah-Ror ARNT and the HPRT house-keeping gene as internal control. a and b, Expression kinetics on days 1, 2, 4, 6, and 8 of culture with 10 nm TCDD. c, Dose-dependency of Ah-R/ARNT expression at 1 and 10 nm TCDD (T 1 and T 10) on day 8 of culture. Shown are original autoradiographs or the calculated index of target gene over HPRT (see the text for details). N, Negative control without DNA in PCR.
Relative expression levels of cytokine genes in FTOC
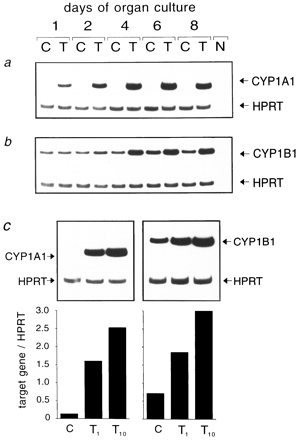
TCDD induces CYP1A1 and CYP1B1 expression in FTOC.
Induction of CYP1A1 (RNA and protein) is considered a hallmark of TCDD action in different species and is known to be Ah-R dependent (26). CYP1A1 induction may therefore be used as a positive control for our system. We tested whether TCDD also induces CYP1A1 mRNA in the FTOCs. As expected, the results of the RT-PCR revealed a strong time- and dose-dependent induction of CYP1A1 mRNA by TCDD, whereas unexposed thymi contained no detectable CYP1A1 mRNA (Fig. 2). The increase by TCDD was detectable as early as on day 1 of culture and continued to increase until day 8. At that time, expression reached ∼50-fold that of controls as quantified densitometrically. We also investigated expression of CYP1B1 mRNA, another Ah-R-inducible gene, in fetal thymi after TCDD exposure. Interestingly, we found that CYP1B1 mRNA is expressed at a constitutive level in fetal thymus (i.e., even in the absence of TCDD). However, this basal level was increased by TCDD (Fig. 2), beginning after a lag period of ∼2 days. Both CYP1A1 and CYP1B1 were induced in a dose-dependent fashion (see Fig. 2). Thus, cells from fetal thymus are not different from other cell types, such as hepatocytes, in their TCDD susceptibility, as measured by induction of transcription of appropriate genes. Moreover, TCDD induction kinetics can differ depending on the respective genes.
FTOCs of 15-day-old embryos were treated with TCDD (T) or solvent alone (C). mRNA was prepared, reverse transcribed and amplified by PCR for CYP1A1 or CYP1B1 and the HPRT house-keeping gene as internal control. a and b, Expression kinetics on days 1, 2, 4, 6, and 8 of culture with 10 nm TCDD. c, Dose-dependency of CYP450 expression at 1 and 10 nm TCDD (T 1 andT 10) on day 8 of culture. Shown are original autoradiographs or the calculated indices of target gene overHPRT (see text for details). N, Negative control without DNA in PCR.
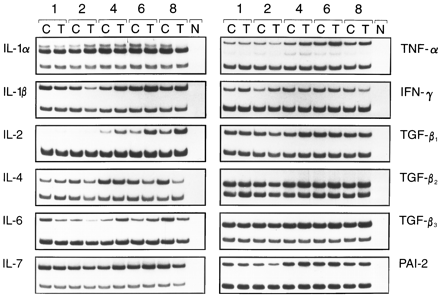
Regulation of cytokine gene expression mediated by TCDD in FTOC.
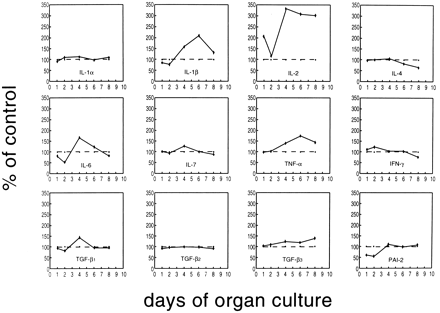
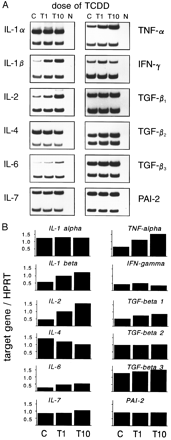
A panel of cytokines was tested for inducibility in thymus cells by TCDD. FTOCs were exposed to TCDD for ≤8 days, and cytokine mRNA levels were determined by RT-PCR on days 1, 2, 4, 6, and 8 of culture. For the preparation of mRNA, thymi of two to four independent FTOCs were pooled. At day 15 of gestation, one thymus lobe contains ∼1 × 105 thymocytes, and then the amount increases to 3–10 × 105 cells within 6–8 days, depending on the presence or absence of TCDD (data not shown). HPRT gene expression was used to compare cDNA levels of a given cytokine between the different days. As shown in Table 2, the basal expression levels of different cytokine mRNAs may be grouped according to the number of amplification cycles run before dropping in the HPRT primers. The results of one of two or three PCR kinetics experiments are summarized in Figs. 3 and 4. TNF-α, IL-1β, and IL-2 are up-regulated compared with the control levels of untreated thymi/thymocytes. The increase starts between days 2 and 3 of the organ culture. In the case of IL-2, the increased expression level is kept until the end of culture, whereas for IL-1β and TNF-α, the mRNA content in the thymi drops again after day 6. In absolute terms, the expression of IL-2 was much lower than that for IL-1β and more so for TNF-α, considering that IL-2 and IL-1β had been amplified for 10 cycles before the start of HPRT amplification, but TNF-α has been amplified for only 3 cycles (see Table 2).
Autoradiographs of amplified mRNA from fetal thymus of 15-day-old embryos organ cultured for 1, 2, 4, 6, and 8 days. Thymi were exposed for the entire culture period to 10 nm TCDD (T) or solvent alone (C). Top band, PCR product of the indicated cytokine. Bottom band, from HPRT, amplified in the same tube as internal standard. N, Negative control without DNA in PCR.
Expression of various cytokine genes under TCDD exposure (10 nm) compared with the expression level with solvent alone (dotted line) during 8 days of FTOC, which was set as 100%. The level of the cytokine by TCDD was calculated in comparison, according to the densitometric scanning data of the autoradiographs (see text for details). Fetal thymi were organ cultured for ≤8 days.
IL-6 expression is down-regulated by day 2 compared with the untreated controls and then up-regulated by day 4; it returns to control levels on days 6 and 8. IL-4 mRNA expression is dropped by day 8 of culture to about half of the control levels, and for TGF-β3, a small, continuing increase can be seen. IL-4, IL-6, and TGF-β3 had been preamplified for 10 cycles (i.e., their basal level is comparatively low) (Table 2). The other tested cytokines varied only very slightly compared with the control levels. Several independently performed PCR kinetics yielded similar results.
We also analyzed the dose-dependency of the mRNA induction on day 5 of cultures. As should be expected for a biological effect of a chemical, a modulation by TCDD is indeed dose dependent (Fig. 5).
A, Dose response of cytokine gene expression levels. Fetal thymi were exposed for 5 days to 1 or 10 nmTCDD (T1 and T10) or to solvent alone (C), and mRNA expression was measured by RT-PCR. Bottom band, from HPRT internal standard. N, Negative control without DNA in PCR. B, Calculated indices of target gene over HPRT for each cytokine (see text for details).
Southern blot analysis and sequence analysis.
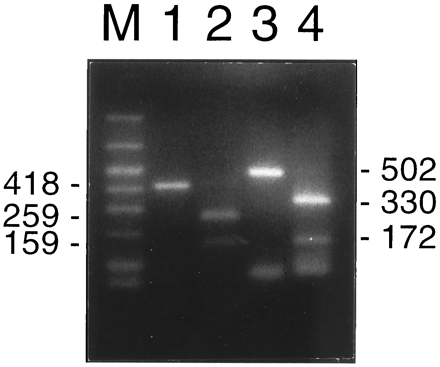
To ensure the specificity of the PCR amplification of selected primers, we digested the PCR products of IL-1β and IL-2 cDNAs with XbaI orHindIII and tested for the generation of fragments of the predicted sizes. In addition, the PCR products of the IL-2 primers were blotted and hybridized to a IL-2-specific probe.
The PCR-amplified 418-bp IL-1β cDNA fragment digested withXbaI should result in two fragments of 259 bp and 159 bp each. The expected subfragments for the 502-bp IL-2 cDNA amplification product digested by HindIII should be 330 and 172 bp. As shown in Fig. 6, the digested fragments were all as expected, confirming that the PCR primers for IL-1β and IL-2 had been specific. Furthermore, in the Southern analysis with an internal IL-2 oligoprobe, the appropriate IL-2 cDNA PCR product lit up. The specificity of PCR products amplified with ARNT-specific primers was confirmed by sequencing (data not shown).
Agarose gel electrophoresis of RT-PCR products from IL-1β (1), its XbaI-restricted fragment (2), IL-2 (3), and itsHindIII cut fragments (4).M, Size marker.
Discussion
During ontogeny, the thymus anlage (i.e., epithelial cells) is colonized by precursors, which eventually give rise to macrophages, interdigitating cells, and lymphocytes (1, 27). Certain surface markers, like the CD4 and CD8 molecules, are progressively expressed in a defined developmental sequence, which is correlated to thymocyte maturity (3, 28). Dioxin interferes with normal thymocyte development in several ways. The progression through the CD4/CD8 maturation stages is faster, more cells appear to audition and pass positive selection, and thymocytes become skewed toward the CD4−CD8+ single-positive thymocyte subset. Moreover, immature thymocytes have a lower proliferation rate, resulting in a lower overall cell count (i.e., thymus atrophy) (17-19,21). These events are mediated mainly by the thymus stroma, as treatment of thymus stroma alone with TCDD suffices to induce such maturation changes (20). The stroma is known to produce a variety of cytokines (4). The time course of intrathymic cytokine production seems to be essential for their role in controlling the thymocyte developmental program. Thus, several studies have been performed to establish the time course and expression strength of cytokines in the developing thymus (5, 29, 30). Moreover, studies in cytokine transgenic mice and in vitro studies in which cytokines were added to thymus organ cultures support a role for cytokines in thymocyte proliferation and/or differentiation and for the properties of the thymic microenvironment. However, the studies in mice, in which distinct cytokines were missing during development due to targeted disruption of the gene, surprisingly yielded opposite results (9). Only IL-7 −/− mice showed significant impairment of T cell development. IL-2 or IL-4 −/− mice or double mutants were normal with respect to T cell development. However, the cytokine network may respond to IL deficiencies by some compensation mechanism. Thus, if cytokine deficiency is of no consequence for a certain developmental process, this need not argue for an irrelevance of overproduction, as found in the present FTOC/TCDD model.
In this study, we have shown through the use of PCR that cytokine expression is modulated in a time-dependent fashion in the fetal developing thymus in the presence of TCDD. One group of cytokines (i.e., IL-1β, IL-2, TGF-β3, and TNF-α) are up-regulated by TCDD; another group (i.e., IL-4, IL-6, and PAI-2) seems to be down-modulated by TCDD; and another group, which includes IL-1β, IL-7, IFN-γ, TGF-β1, and TGF-β2, remains unaffected by TCDD.
We used homogenized thymi for this study, so we do not distinguish between different thymus cell types (stromal cells versus thymocyte subsets) as producers of cytokines. IL-1 and TNF-α transcripts, which are up-modulated by TCDD, were previously detected within the cortex. Both of them are most likely produced by macrophages and immature thymocytes but also to some extent by cortical epithelial cells (31), whereas IL-2, IL-4, and IFN-γ are assumed to be produced by immature thymocytes (5, 6, 32). IL-2 induction was comparatively strongly modulated by TCDD in our FTOCs. Mature CD8 cells were found to be capable of producing IL-2, albeit only after stimulation [e.g., with mitogens (33)], and TCDD skews thymocyte maturation toward CD4−CD8+ cells (18). Recently, it was shown that IL-2 is induced in CD8 cells sorted out from TCDD-exposed FTOCs.2 IL-6 is exclusively produced by epithelial cells. Thus, due to our method of cell preparation, which may favor thymocytes, we may even have underestimated the induction of IL-1, TNF-α, and IL-6 by TCDD. Both thymocytes and thymus stroma express the Ah-R and may therefore have gene transcription induced by TCDD. The Ah-R apparently plays a crucial role in cytokine modulation during thymus ontogeny.
Cytokines might also be involved in the selection events to which thymocytes are subjected. Negative selection eliminates thymocytes with self-antigen specificity, yet positive selection favors thymocytes bearing a TCR that recognizes foreign antigen in the context of self-MHC. We have previously shown that in the presence of TCDD, more CD4+CD8+ thymocytes bear the footprints of positive selection (i.e., express CD69 on their surface) and express the bcl-2 molecule in the cytoplasm, which may protect them from selection-induced apoptosis (21). IL-1 and IL-2 inhibited in vitro the anti-CD3-induced apoptosis (34), which fits well with our observation of more cells passing positive selection and the induction of these cytokines by TCDD.
Studies on irradiated mice that had been adoptively transferred with thymocyte precursors and given cytokines showed that recombinant IL-6 accelerates the differentiation from very immature thymocytes to the double-positive cells. Accelerated differentiation was also observed by us in FTOCs by TCDD during the first days of culture. However, IL-6 is up-modulated by TCDD only after day 3 or 4; thus, it remains to be shown whether accelerated maturation and IL-6 up-regulation are causally related. As pointed out above, IL-6 is mainly produced by the thymus epithelium, so it would be interesting to study cytokine production on carefully sorted thymus cell subpopulations. The addition of IL-1β and TNF-α to FTOCs led to an accumulation of CD4−CD8+ single-positive cells (35, 36), a phenomenon that we also observed after TCDD treatment and that is in accordance with the high up-regulation of IL-1β by TCDD found in this study.
TNF-α added to immature thymocytes (CD24−CD117+) was shown to promote their differentiation and led to expression of CD25, the IL-2 receptor, which is necessary for further progression along the thymocyte maturation pathways (37). Likewise, up-modulation of TNF-α, as occurs with TCDD, may speed up the acquisition of IL-2 receptor and thus maturation. We found a higher proportion of immature cells expressing CD25int in the presence of TCDD (18).
It was beyond the scope of this study to show whether the TCDD-induced cytokine modulation is a direct effect of TCDD (i.e., whether the gene promoters are activated/inactivated by the TCDD/Ah-R complex). Run-off experiments, which must be performed separately for each cytokine, could unequivocally demonstrate this. However, the DREs that were identified in the IL-2 promoter are capable of binding to the Ah-R as shown by band shifts.3By computer analysis, several potential DREs have been found in the promoter regions of most cytokine genes, which are modulated here by TCDD (16).
Direct induction of IL-1β via transcription initiation has previously been found in human keratinocytes (38), and for TGF-β1–3, it was found in a human breast cancer cell line, MCF-7 (39).
Although self-evident, it should be noted that induction of transcription need not translate into induction of protein expression. In addition, post-transcriptional changes may influence the overall transcript stability as a consequence. Protein modulation would have to be separately studied for each IL of interest.
Thymic organ cultures have been extensively used as tools to study and dissect ex vivo the ontogeny of fetal thymi. As judged by comparison with the in utero development, they seem to be a real-life image (40). However, due to their nature, FTOCs may be artifactual for particular parameters; thus, it will always be necessary to repeat and extend studies to the in vivosituation.
In conclusion, we have shown that cytokine production is modulated by TCDD in fetal thymi and that the current knowledge on the effects of the modulated cytokines on thymus development correlates well with our previous observations of the interference by TCDD with thymus development.
Acknowledgments
We are grateful to M. Schneider for critical reading of the manuscript and helpful comments. We thank C. Vogel and J. Abel for providing primers, and Z. Ran for sequencing. Special thanks go to S. Steinwachs for expert technical help, management of the mouse breeding colony, and skillful artwork.
Footnotes
- Received February 10, 1997.
- Accepted March 27, 1997.
-
Send reprint requests to: Dr. Charlotte Esser, Medical Institute of Environmental Hygiene, Auf’m Hennekamp 50, 40225 Düsseldorf, Germany. E-mail:chesser{at}uni-duesseldorf.de
-
↵1 Current affiliation: SUNY Health Science Center, Syracuse, New York 13210.
-
↵2 Myung-Shin Jeon, personal communication.
-
This work was supported financially through Sonderforschungsbereich 503, Molecular and Cellular Mediators of Exogenous Noxes (Heinrich-Heine-University of Düsseldorf, Düsseldorf, Germany).
-
↵3 Myung-Shin Jeon, personal communication.
Abbreviations
- Ah-R
- aryl hydrocarbon receptor
- ARNT
- aryl hydrocarbon receptor nuclear transporter
- DRE
- dioxin-responsive element
- FTOC
- fetal thymus organ culture
- RT
- reverse transcription
- PCR
- polymerase chain reaction
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- TCR
- T cell receptor
- IL
- interleukin
- TNF-α
- tumor necrosis factor-α
- TGF-β
- tumor growth factor-β
- IFN-γ
- interferon-γ
- PAI-2
- plasminogen activator inhibitory factor
- The American Society for Pharmacology and Experimental Therapeutics