Abstract
We tested the hypothesis that the human β1-adrenergic receptor displays constitutive activity and that β-adrenergic antagonists differ in their ability to modulate this constitutive activity. Transfection of the cDNAs of the human β1- and β2-adrenergic receptors into COS-7 cells caused increases in basal cAMP that were proportional to the receptor levels, thus demonstrating constitutive activity for both subtypes. At comparable receptor levels, the increase in basal cAMP was about 5-fold higher for the β2- than for the β1-subtype. As a model for enhanced β-adrenergic signaling at the whole-organ level, we used transgenic mice with heart-specific overexpression of the human β1-adrenergic receptor. In this model, the β1-adrenergic receptor displayed constitutive activity as evidenced by a higher spontaneous beating rate of isolated right atria from β1-transgenic versus wild-type mice. This difference was abolished by the addition of CGP20712A, demonstrating inverse agonist properties of this compound. We then tested whether various β-adrenergic antagonists currently in clinical use for the treatment of heart failure differ in their ability to modulate constitutive activity of the cardiac β1-adrenergic receptor. The β1-selective antagonists metoprolol and bisoprolol showed significant inverse agonist activity at the β1-adrenergic receptor. Carvedilol behaved as a neutral antagonist and xamoterol displayed marked partial agonist activity. We conclude that the human β1-adrenergic receptor displays constitutive activity that is considerably lower than that of the β2-subtype. β-Adrenergic antagonists currently in clinical use differ in their ability to exert inverse agonist activity at the human β1-adrenergic receptor, which may contribute to their therapeutic effects.
Activation of cardiac β-adrenergic receptors plays a central role in regulating the physiological responses of the heart to an increased demand (Brodde and Michel, 1999). Chronic activation of cardiac β-adrenergic receptors occurs in heart failure because of an increase in sympathetic activity and circulating catecholamine levels (Chidsey and Braunwald, 1966). Although this adaptive response to compensate for the heart's inability to meet hemodynamic demands has traditionally been appreciated as positive inotropic support, the perception of this phenomenon has changed within the past decade. Several lines of evidence now indicate that the chronic sympathetic activation seen in heart failure is detrimental and indeed plays an important part in the progression of this disease. Myocardial toxicity of infused catecholamines has been demonstrated both in animal studies and in humans (Rona, 1985). Recently, chronic heart-specific activation of β1-adrenergic receptors in a transgenic animal model has been shown to cause myocyte hypertrophy, myocardial fibrosis, and eventually heart failure (Engelhardt et al., 1999; Bisognano et al., 2000). Several large clinical trials with β-adrenergic receptor antagonists have demonstrated a significant benefit of this therapeutic principle (Australia/New Zealand Heart Failure Research Collaborative Group, 1997; CIBIS-II, 1999; MERIT-HF, 1999). Thus, within the past decade a paradigm shift has occurred, from inotropic support toward pharmacological suppression of sympathetic activation (Bristow, 2000a).
However, the substances among the available β-adrenergic receptor antagonists that provide the greatest benefit in the setting of heart failure remain unclear (Bristow, 2000b) . One general property that contributes to the therapeutic effects of receptor antagonist drugs is their ability to modulate the activation state of the receptor. G-protein-coupled receptors display different degrees of constitutive activity (de Ligt et al., 2000). Among the adrenergic receptors, the β2-adrenergic receptor has been found to possess considerable constitutive activity and has been used as one of the prototypical receptors to develop the concept of inverse agonism. These experiments have been carried out in vitro with reconstituted systems (Freissmuth et al., 1991) and after transfection of receptors in cell culture (Adie and Milligan, 1994; Chidiac et al., 1994) and have more recently been extended to transgenic animals (Bond et al., 1995; Zhou et al., 1999a,b). Transgenic mice with 200-fold overexpression of the human β2-adrenergic receptor displayed a marked increase in basal tension and beating frequency of isolated atria compared with wild-type animals. This increase was inhibited by 70% upon the addition of ICI-118,551, acting as an inverse agonist at this receptor.
In the present study, we assessed the intrinsic activity of both β1- and β2-adrenergic receptors. We then determined the effects of propranolol, metoprolol, bisoprolol, CGP 20712A, carvedilol, and xamoterol on the activation level of the β1-adrenergic receptor in atria from transgenic mice with cardiac overexpression of human β1-adrenergic receptors.
Materials and Methods
Cell Culture and Transfection.
COS-7 cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal calf serum and transfected with the cDNA for the human β1- or β2-adrenergic receptors under the control of the cytomegalovirus promotor by the DEAE-dextran method (Ausubel et al., 1997). The total amount of transfected DNA varied from 0.001 to 5 μg/plate. Twenty-four hours after transfection, cells were split, and assays were done 48 h after transfection.
Determination of cAMP Levels.
48 h after transfection, cells were washed twice with HEPES buffer (137 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 20 mM HEPES, pH 7.3) and resuspended in the same buffer with 0.5 mM 3-isobutyl-1-methylxanthine. The cells were incubated for 20 min at 37°C and the reaction was stopped by addition of boiling water. Cellular cAMP was determined by radioimmunoassay (Immunotech, France).
Radioligand Binding Studies.
Cell membranes were prepared by lysis of the cells in 5 mM Tris-HCl, 5 mM EDTA, pH 7.4, centrifugation at 1,000g for 10 min (4°C), and centrifugation of the supernatants at 50,000g for 15 min (4°C). The pellets were resuspended in 75 mM Tris-HCl, 12.5 mM MgCl2, and 1 mM EDTA, pH 7.4. For radioligand binding assays, 20 μg of membrane protein were incubated with various concentrations of [3H] CGP12177 (44 Ci/mmol) (up to 5 nM). Nonspecific binding was assessed in the presence of 1 μM (−)-propranolol. Incubations were terminated by filtration through GF/C filters (Whatman, Clifton, NJ).
Transgenic Animals.
The generation of the transgenic mouse line β1TG4 overexpressing the human β1-adrenergic receptor has been described elsewhere (Engelhardt et al., 1999). Briefly, transgenic mice were obtained by pronuclear injection of fertilized oocytes from FVB/N mice with a transgenic construct harboring the coding sequence of the human β1-adrenergic receptor under the control of the murine α-myosin heavy chain (αMHC) promotor. Mice were used at the age of 3 months. As expected from the expression characteristics of the αMHC promotor, receptor levels were higher at this age than at the age of 6 weeks studied in the original report (Engelhardt et al., 1999) and were determined at 2.7 ± 0.3 pmol/mg of membrane protein. Screening for integration of the transgene was carried out by PCR with a sense primer 5′-AGG ACT TCA CAT AGA AGC CTA G-3′ located in the αMHC-promoter and an antisense primer 5′-TGT CCA CTG CTG AGA CAG CG-3′, located in the β1-receptor coding sequence. All mice were kept in a specified-pathogen-free facility. Investigation of these mice was approved by the local animal experimentation and gene technology authorities (protocol number Az 621-2531.01–26/98, University of Würzburg).
Organ Bath Experiments.
Hearts were excised and placed in carbogenated modified Tyrode's solution (119 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, 22.6 mM NaHCO3, 0.42 mM NaH2PO4, 0.025 mM EDTA, 10 mM glucose, 0.2 mM ascorbic acid, pH 7.4). Right atria were dissected and placed in a fresh dissection plate. Atria were then tied with two 6–0 silk sutures and placed in a carbogenated 35°C tissue bath with modified Tyrode's solution. Before the addition of the pharmacological substances (1 μM) the content of the organ baths was changed five times with 5 min intervals between the individual washing steps. The atria were allowed to contract spontaneously. Signals from isometric force transducers (FMI, Seeheim, Germany) were fed via a bridge amplifier to a PowerLab system (A. D. Instruments, Castle Hill, Australia) and atrial beating frequency was recorded continuously throughout the experiment. To deplete animals of endogenous catecholamines, we treated transgenic animals with reserpine (5 mg/kg s.c. 24 h before the experiment and 2.5 mg/kg 3 h before the experiment) or vehicle (dimethyl sulfoxide). Tissue noradrenaline levels were assessed from left ventricular homogenates by HPLC as described previously (Graefe et al., 1997).
Statistical Analysis.
Average data are presented as mean ± S.E.M. Statistical analyses (t tests for pairwise comparisons or analysis of variance) were used where appropriate with the InStat software package (GraphPad, San Diego, CA). Differences were considered significant when p < 0.05.
Results
β1- and β2-Adrenergic Receptors Show Different Levels of Constitutive Activity.
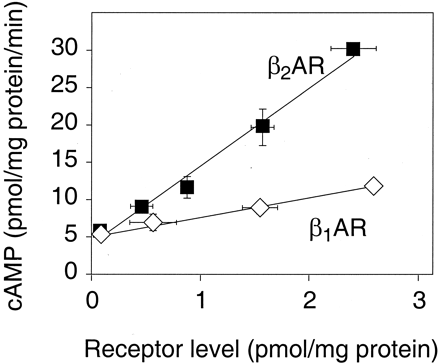
To study the constitutive activity of the human β-adrenergic receptor subtypes, the cDNAs of the β1 and β2 subtypes were transiently transfected into COS-7 cells and cAMP levels were determined. For both receptor subtypes, an increase in basal cAMP levels was detected that was dependent on the amount of cDNA transfected and on the resulting receptor levels (Fig. 1). However, the extent to which the transfected receptors increased basal cAMP levels was markedly different between the individual subtypes. The increase of basal cAMP levels was about 5-fold higher for the β2- than for the β1-subtype of the human β-adrenergic receptor (Fig. 1). These results show that the β1-adrenergic receptor does possess constitutive activity, but it is considerably lower than that of the β2-subtype. We then asked whether the small constitutive effects of the β1-adrenergic receptor in COS-7 cells might be exploited to search for inverse agonist effects of β-adrenergic antagonists. Various agents were added at saturating conditions to COS-7 cells transiently expressing the human β1-adrenergic receptor. Of these, only CGP20712A produced a small decrease in cAMP (10 ± 3%). However, this decrease was too small to permit studies that are more detailed.
cAMP accumulation as a function of receptor density in COS-7 cells transfected with the cDNA for the human β1- or β2-adrenergic receptor. Various amounts of plasmids containing the cDNAs for the human β1- and β2-adrenergic receptor under the control of the CMV promoter were transfected into COS-7 cells. After 2 days, receptor densities were determined by radioligand binding and cAMP-levels were determined by radioimmunoassay. Each point represents mean ± S.E.M. of three to six independent experiments.
Constitutive Activity of the Human β1-Adrenergic Receptor in Transgenic Mice.
We then sought to compare potential differences in inverse agonist activity of clinically used β-adrenergic receptor antagonists in a more physiological model of enhanced β-adrenergic receptor signaling. We used transgenic mice with heart specific overexpression of the human β1-adrenergic receptor and measured spontaneous beating frequency of isolated right atria compared with that of wild-type littermates (Engelhardt et al., 1999).
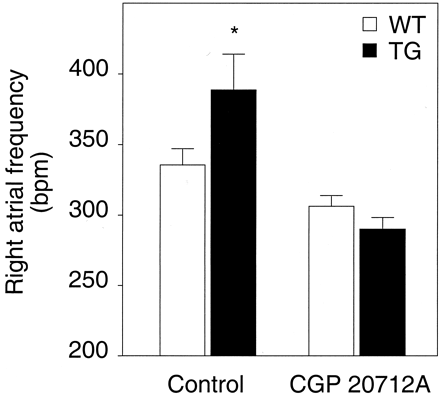
In β1-transgenic mice, we found a significantly higher spontaneous frequency of isolated right atria compared with nontransgenic mice (Fig. 2). Right atria from transgenic animals displayed a 16% higher spontaneous beating frequency. After addition of the β1-selective compound CGP20712A, this difference disappeared completely (Fig. 2). This was because CGP20712A caused a ≈25% decline of the frequency in transgenic atria, but only a small decline in wild-type atria. The effect of CGP20712A could be prevented by the simultaneous addition of propranolol to the organ bath (data not shown).
Effect of CGP20712A on the increased spontaneous beating rate in right atria from β1-adrenergic receptor transgenic mice. CGP20712A acts as an inverse agonist at the human β1-adrenergic receptor. Right atrial frequencies were recorded before and 60 min after addition of 1 μM CGP. Data are means ± S.E.M. from 8 to 10 independent experiments. *p < 0.05 wild-type versus β1-transgene.
We next sought to determine whether the increased frequency of transgenic atria was caused by constitutive activity of the unoccupied receptors or if endogenously released catecholamines acting on the overexpressed receptors might contribute to the higher spontaneous beating rate of the transgenic atria and thus confound our measurements. To deplete animals of endogenous catecholamines, we treated transgenic animals with reserpine (5 mg/kg s.c. and 2.5 mg/kg, respectively, 24 h and 3 h before the experiment) or vehicle (dimethyl sulfoxide). The reserpine treatment caused a depletion of cardiac noradrenaline by more than 99,9% as assessed by determination of tissue catecholamine levels by HPLC (Fig.3A). The treatment of transgenic animals with reserpine, however, had no significant impact on the spontaneous beating frequency of isolated right atria compared with atria from nonreserpinized animals (Fig. 3B). We conclude that the contribution of endogenous catecholamines to the high frequencies of isolated atria from transgenic animals is negligible and, consequently, that they were indeed caused by constitutive activity of the overexpressed β1-adrenergic receptors.
Effects of reserpine on cardiac noradrenaline levels and spontaneous right atrial frequency in β1-adrenergic receptor transgenic mice. A, to determine whether endogenously released catecholamines were present during the experiments, mice were reserpinized as described underMaterials and Methods and tissue noradrenaline levels were determined by HPLC analysis. Two representative HPLC tracings from a reserpinized animal and a control animal are shown. B, spontaneous right atrial frequencies of atria from reserpinized and nonreserpinized animals were monitored for the duration of a standard experiment (90 min, as in Fig. 2). There was no significant difference between the two groups, indicating that with the assay conditions used, significant amounts of catecholamines were not present. Data represent means ± S.E.M. from three independent experiments.
Because of the lack of effect of the reserpine treatment on the frequency of isolated atria, the following experiments were carried out with an extensive washing schedule before substance addition (seeMaterials and Methods) but without reserpinizing the animals.
Various β-Adrenergic Receptor Antagonists Differ in Their Ability to Regulate the Activation Level of Cardiac β1-Adrenergic Receptors.
Because the higher frequency seen in atria from transgenic animals compared with atria from nontransgenic animals seemed to be caused by constitutive activity and could be abolished by CGP20712A, this type of experiment seemed to provide a physiological model to screen for inverse agonist effects of compounds at β1-adrenergic receptors.
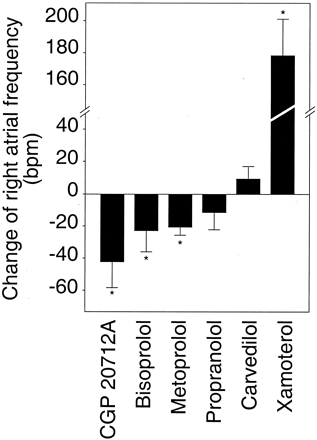
To test the hypothesis that different β-adrenergic antagonists currently used in clinical practice might differ in their ability to alter the basal activation level of the human β1-adrenergic receptor, the effects of these antagonists on the spontaneous beating frequency of isolated right atria from β1-transgenic mice were determined (Fig. 4). The β1-selective antagonists CGP20712A, bisoprolol, and metoprolol all behaved as inverse agonists in this model of enhanced β1-adrenergic receptor signaling. Propranolol caused only a small and statistically insignificant decrease of the spontaneous beating rate of right transgenic atria. The nonselective β-adrenergic receptor antagonist carvedilol did not display inverse agonist activity at the β1-adrenergic receptor; rather, it caused a slight, statistically insignificant stimulation. Finally, xamoterol showed partial agonist activity, as evidenced by an increase of right atrial frequency of 178 ± 25 bpm compared with unstimulated atria.
Change of right atrial frequency under treatment with various β-adrenergic receptor antagonists. Right atria from β1-adrenergic receptor transgenic mice were investigated as in Fig. 2 in the presence or absence (control) of various β-adrenergic antagonists (1 μM). Sixty minutes after drug addition, the change of the beating rate was determined and corrected for the spontaneous decrease observed in controls (transgenic atria with vehicle alone). Data are means ± S.E.M. from 5 to 12 experiments for each drug. *p < 0.05.
Discussion
We used two complementary approaches to assess whether the human β1-adrenergic receptor displays constitutive activity and, if so, to what extent clinically used β-adrenergic antagonists can modify this level of basal receptor activation: overexpression of both receptor subtypes by transient transfection in COS-7 cells and transgenic overexpression in the mouse heart. Three major findings of this study are: 1) The human β1-adrenergic receptor displays constitutive activity. 2) The constitutive activity of the β1-adrenergic receptor is considerably lower than that of the β2 subtype. 3) β-Adrenergic antagonists currently in clinical use differ in their ability to exert inverse agonist activity at the human β1-adrenergic receptor.
Although constitutive activity of various receptors, notably the β2-adrenergic receptor, is well documented, only a few studies have attempted to study constitutive activity of the human β1-adrenergic receptor. There are some indications that β-adrenergic antagonists may display inverse agonist activity in vivo (reviewed by Bristow 2000a), but these effects are difficult to quantify because they always occur in the presence of endogenous catecholamines. In isolated human cardiomyocytes, β-adrenergic receptors have been shown to activate sarcolemmal Ca2+-channels in the absence of agonist (Mewes et al., 1993). Recently, constitutive activity of β-adrenergic receptors has been demonstrated in rat and human cardiac tissue (Varma et al., 1999; Maack et al., 2000). However, approximately 30% of the β-adrenergic receptors on rat and human cardiomyocytes are of the β2-subtype (Brodde and Michel, 1999). Because the β2-subtype displays a much higher degree of constitutive activity (see below), it may lead to an overestimation of the actual constitutive activity of the β1-subtype if a small part of the β2-receptor is blocked in experiments with β1-adrenergic antagonists. Such a potential contribution of β2-receptors (e.g., by application of a highly β2-selective antagonist, such as ICI-118,551) has not been tested in experiments with human cells or tissue.
In contrast to these models, atrial tissue from transgenic mice with overexpression of the human β1-adrenergic receptor can be assumed to be virtually free of functionally active β2-receptors, because β1-adrenergic receptor knockout mice do not show significant contractile responses to isoprenaline (Rohrer et al., 1996) and because the β1-component is enhanced. Indeed, with this β1-specific model, we observed only a low level of constitutive activity, which was clearly smaller than that seen in studies on tissues in which both receptor subtypes are functionally relevant (Mewes et al., 1993; Varma et al., 1999; Maack et al., 2000). Most recently, Zhou et al. (2000) reported only marginal increases of the contraction amplitude after adenoviral transfection of isolated cardiomyocytes with the β1-adrenergic receptor. After addition of CGP20712A to their cell culture, they observed only a small, insignificant decrease of the contraction amplitude. There are several reasons which might account for the observed differences: 1) The transfected cardiomyocytes studied by Zhou et al. expressed markedly lower receptor levels than our model (0.6 pmol/mg of membrane protein versus 2.7 ± 0.3 pmol/mg of membrane protein). 2) Zhou et al. (2000) used the contraction amplitude as a parameter, although we studied the spontaneous frequency of isolated right atria. 3) Finally, the different models studied might affect the magnitude of the observed effect. We studied intact organ physiology in transgenic mice, whereasZhou et al. (2000) looked at the effects of acute (i.e., within days) overexpression of β1-adrenergic receptors in isolated cells. It seems that the increased frequency of β1-adrenergic receptor transgenic atria is a particularly sensitive tool for detecting and measuring constitutive activity of these receptors and its inhibition by inverse agonists, because this effect is very reproducible and can be measured with high accuracy.
A comparison of our data with those reported for the β2-receptor transgenic mice (Milano et al., 1994; Bond et al., 1995) shows a much lower constitutive activity of the β1-subtype compared with the β2-subtype. This difference is also apparent from the in vivo heart rate, which was only moderately elevated in β1-receptor transgenic mice (Engelhardt et al., 1999), but massively in β2-receptor transgenic mice (Milano et al., 1994; Bond et al., 1995). However, these in vivo measurements are confounded by the influence of endogenous catecholamines.
A similar difference between the constitutive activities of the two receptor subtypes was seen in transfected cell lines. At comparable levels of transient expression in COS-7 cells, basal cAMP levels were about 5-fold higher for the β2-subtype than for the β1-subtype. We must assume, therefore, that the level of constitutive activity of the human β1-adrenergic receptor is considerably lower than that of the β2-subtype. Despite the low level of constitutive activity of β1-adrenergic receptor, this property might become important when the signaling system is sensitized either experimentally [for example with forskolin (Mewes et al., 1993; Maack et al., 2000)] or pathologically, or in situations in which β1-specific signaling further aggravates preexisting damage of affected tissues. Recently,Mason et al. (1999) reported on a variant of the β1-adrenergic receptor (Arg 389), which displays enhanced coupling to Gs proteins. It will be interesting to study this mutant for constitutive activity and the effects of inverse agonists. Point mutants of the β1-adrenergic receptor at position 322 have been generated that display enhanced constitutive activity (Lattion et al., 1999). At these mutants, CGP, betaxolol, and metoprolol displayed inverse agonistic activity, but the β2-selective antagonist ICI-118,551 also showed inverse agonistic activity.
We used the β1-receptor transgenic atria as a physiological model to search for inverse agonist effects. Both metoprolol and bisoprolol displayed significant inverse agonist activity almost comparable with the experimental substance CGP20712A. In contrast, xamoterol displayed clear partial agonist activity. The difference between these two groups correlates well with the results obtained in clinical trials for heart failure. Both for bisoprolol and for metoprolol, benefits in mortality from heart failure patients have been demonstrated in large clinical trials (CIBIS-II, 1999; MERIT-HF, 1999). In contrast, xamoterol led to an increase in mortality (Xamoterol in Severe Heart Failure Study Group, 1990). These data suggest that β1-mediated signaling in a diseased heart, even at reduced or very low levels, is generally detrimental. This notion is also supported by the fact that chronically enhanced β1-receptor mediated signaling is sufficient to cause hypertrophy and ultimately heart failure in the mouse model used for the present study (Engelhardt et al., 1999). It seems reasonable to predict from these data that in the treatment of heart failure, most compounds with partial agonist activity are detrimental.
The differences in inverse agonist activities between the compounds that have been proven to be useful in heart failure (i.e., bisoprolol, metoprolol and carvedilol) were rather small. Although bisoprolol and metoprolol had significant inverse agonistic effects, carvedilol was devoid of inverse agonistic activity. Clinical studies comparing directly the efficacy of these compounds in heart failure are not yet available; thus there are no data that would allow a correlation between inverse agonist properties and their clinical usefulness. Such interpretations would be further complicated by the fact that carvedilol is also a potent α1-adrenergic receptor antagonist and has significant antioxidative properties (Feuerstein et al., 1997; Dandona et al., 2000). Both mechanisms of action have been implicated in its favorable effect.
Even though the level of constitutive activity of the β1-adrenergic receptor is rather small, such small differences in long-term activation of β1-adrenergic receptors may be important for cardiac function. This can be deduced from the observation that autoantibodies against the β1-adrenergic receptor have only small intrinsic activity at these receptors but are nevertheless associated with decreased cardiac function (Jahns et al., 1999; Wallukat et al., 1999) and their removal is clinically beneficial (Muller et al., 2000).
In summary, our data show that the human β1-adrenergic receptor has constitutive activity, albeit to a lesser extent than the β2-subtype. The type of mouse used here should be a valuable tool for testing upcoming β-adrenergic receptor antagonists with respect to their inverse agonist activity at the human β1-adrenergic receptor in a physiological model. Future experimental and clinical studies are necessary to estimate the contribution of inverse agonist activities to the therapeutic effects of β1-adrenergic receptor-blocking drugs.
Acknowledgments
We thank Carsten Arnolt and Karl-Heinz Graefe for determination of myocardial noradrenaline levels.
Footnotes
-
These studies were supported by grants from the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and the European Union.
- Abbreviations:
- αMHC
- α myosin heavy chain
- HPLC
- high-performance liquid chromatography
- Received February 5, 2001.
- Accepted June 1, 2001.
- The American Society for Pharmacology and Experimental Therapeutics