Abstract
Recent clinical studies reveal that selective agonists of group II metabotropic glutamate (mGlu) receptors have robust efficacy in treating positive and negative symptoms in patients with schizophrenia. Group II mGlu receptor agonists also modulate the in vivo activity of psychotomimetic drugs and reduce the ability of psychotomimetic hallucinogens to increase glutamatergic transmission. Because increased excitation of the medial prefrontal cortex (mPFC) has been implicated in pathophysiology of schizophrenia, the ability of group II mGlu receptor agonists to reduce hallucinogenic drug action in this region is believed to be directly related to their antipsychotic efficacy. A novel class of ligands, termed positive allosteric modulators, has recently been identified, displaying exceptional mGlu2 receptor selectivity. These compounds do not activate mGlu2 receptors directly but potentiate the ability of glutamate and other agonists to activate this receptor. We now report that the mGlu2 receptor-selective positive allosteric modulator biphenyl-indanone A (BINA) modulates excitatory neurotransmission in the mPFC and attenuates the in vivo actions of the hallucinogenic 5-HT2A/2C receptor agonist (-)2,5-dimethoxy-4-bromoamphetamine [(-)DOB]. BINA attenuates serotonin-induced increases in spontaneous excitatory postsynaptic currents in the mPFC, mimicking the effect of the mGlu2/3 receptor agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV). In addition, BINA reduced (-)DOB-induced head twitch behavior and Fos expression in mPFC, effects reversed by pretreatment with the mGlu2/3 receptor antagonist 2S-2-amino-2-(1S,2S-2-carboxycyclopropan-1-yl) -3 - (xanth-9-yl-)propionic acid (LY341495). These data confirm the relevance of excitatory signaling in the mPFC to the behavioral actions of hallucinogens and further support the targeting of mGlu2 receptors as a novel strategy for treating glutamatergic dysfunction in schizophrenia.
Psychotomimetic drugs, including dissociative anesthetics (phencyclidine and ketamine) and hallucinogens (lysergic acid diethylamide and psilocybin), are commonly used in experimental models of psychosis to probe the pathophysiology of schizophrenia. In humans, these drugs produce a state of drug intoxication that resembles many of the symptoms of acute schizophrenia (Vollenweider and Geyer, 2001). In addition, human brain imaging demonstrates a common pattern of increased activity in the frontal cortex caused by psychotomimetic drugs and acute schizophrenia (Cleghorn et al., 1989; Hermle et al., 1992; Kaplan et al., 1993; Ebmeier et al., 1995; Vollenweider et al., 1997a,b), suggesting a potential role of the excitatory neurotransmitter glutamate in psychotic behaviors. The release of glutamate from nerve terminals is negatively regulated by group II mGlu receptors, mGlu2 and mGlu3 subtypes. Recent evidence that the behavioral effects of ketamine in humans are disrupted by an mGlu2/3 receptor agonist (Krystal et al., 2004) supports the targeting mGlu2/3 receptors as a therapeutic target for schizophrenia. Consistent with this hypothesis, data from a recent phase II clinical trial shows that an mGlu2/3 receptor agonist is an effective antipsychotic therapy, comparable with the atypical antipsychotic drug olanzapine (Schoepp, 2006). These studies demonstrate the therapeutic potential of mGlu2/3 receptors and indicate the need to further investigate the individual roles of mGlu2 and mGlu3 receptors in behavior and glutamatergic neurotransmission.
Investigation of hallucinogenic drug action as a model of psychosis suggests that the behavioral effects of hallucinogens in humans are mediated by activation of serotonin (5-HT) subtype 2A (5-HT2A) receptors (Vollenweider et al., 1998). Hallucinogen-induced activation of 5-HT2A receptors increases spontaneous excitatory postsynaptic currents (EP-SCs) in layer V pyramidal neurons of the mPFC (Aghajanian and Marek, 1997, 1999), consistent with the induction of glutamate release from presynaptic nerve terminals. Extensive electrophysiological experiments demonstrate a physiological antagonism between 5-HT2A receptors and mGlu2/3 receptors in mPFC (Marek et al., 2000), with activation of mGlu2/3 receptors dramatically attenuating 5-HT2A receptor-induced EPSCs, supporting the use of hallucinogens as a model for studying the regulation of hyperactive glutamatergic signaling by mGlu2/3 receptors in vivo. Consistent with this model, mGlu2/3 receptor agonists attenuate behavioral effects of hallucinogens (Gewirtz and Marek, 2000; Klodzinska et al., 2002; Winter et al., 2004) and dissociative anesthetics (Moghaddam and Adams, 1998; Cartmell et al., 1999; Galici et al., 2005; Greco et al., 2005).
Although preclinical and clinical studies clearly illustrate that group II mGlu receptors are viable antipsychotic targets, conclusions are hindered by the lack of selectivity of all available pharmacological tools for the individual group II mGlu receptor subtypes. A novel class of mGlu2 receptor-selective ligands, known as positive allosteric modulators, has recently been developed and shown to have in vivo properties in behavioral assays of antipsychotic and anxiolytic activity that mimic mGlu2/3 receptor agonists (Johnson et al., 2003, 2005; Galici et al., 2005, 2006). Positive allosteric modulators do not activate the receptor directly but act at a site separate from the glutamate binding site to potentiate the agonist response, inducing a leftward shift in the glutamate concentration-response curve (Johnson et al., 2003, 2005; Schaffhauser et al., 2003; Galici et al., 2006). Biphenyl-indanone A (BINA), a recently characterized, positive allosteric modulator of mGlu2 receptors with in vivo activity, is highly selective for mGlu2 receptor over other mGlu receptor subtypes (Galici et al., 2006). The current study takes advantage of BINA to test the ability of selective potentiation of mGlu2 receptors to attenuate the actions of the hallucinogenic 5-HT2A receptor agonist (-)2,5-dimethoxy-4-bromoamphetamine (DOB).
Materials and Methods
Brain Slice Electrophysiology
Brain slices were prepared from Sprague-Dawley rats (postnatal day 16-24). Rats were anesthetized with isoflurane. The brain was rapidly removed and submerged in ice-cold modified oxygenated artificial cerebrospinal fluid (ACSF) composed of 230 mM sucrose, 2.5 mM KCl, 0.5 mM CaCl2, 10 mM MgSO4, 1.25 mM NaH2PO4,26 mM NaHCO3, and 10 mM d-glucose. Coronal brain slices (300 μm) containing the mPFC were cut using a Leica VT1000S vibratome (Leica Microsystems, Nussloch, Germany). Slices were incubated in oxygenated ACSF at 32°C for 1 h and maintained at room temperature until transferred to a recording chamber. The recording chamber was continuously perfused at ∼30°C with oxygenated ACSF containing 126 mM NaCl, 2.5 mM KCl, 3.0 mM CaCl2, 2.0 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 10 mM d-glucose.
Spontaneous EPSCs were recorded from layer V pyramidal cells in whole-cell voltage-clamp mode using an Axon Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and visualized with an Olympus BX50WI upright microscope (Olympus, Lake Success, NY) coupled with a 40× water immersion objective and Hoffman optics. Borosilicate glass (World Precision Instruments, Sarasota, FL) patch pipettes were prepared using a Flaming-Brown micropipette puller (Model P-97; Sutter Instruments, Novato, CA) and filled with 123 mM potassium gluconate, 7 mM KCl, 1 mM MgCl2, 0.025 mM CaCl2, 10 mM HEPES, 0.1 mM EGTA, 2 mM ATP, and 0.2 mM GTP at a pH of 7.3 and osmolarity of ∼295 mOsM. Filled patch pipettes had resistances of 2 to 3 MΩ. EPSCs were recorded at a holding potential of -70 mV; GABAA receptor-mediated inhibitory currents were undetectable under these conditions. The voltage-clamp signal was low-pass-filtered at 2 kHz, digitized at 10 kHz, and acquired using a Clampex9.2/DigiData1332 system (Molecular Devices). All drugs were bath-applied. After a stable baseline was recorded for 5 to 10 min, the effect of BINA or DCG-IV on baseline EPSCs or 5-HT-induced EPSCs (applied 3 min after 5-HT) was examined.
In Vivo Studies
Subjects. (-)DOB-induced head twitch response and Fos expression were examined in male ICR(CD-1) mice (Harlan, Indianapolis, IN) at 8 to 10 weeks of age. Mice were housed in groups of four or five animals per cage in a large colony room on a 12:12 light/dark cycle with light onset at 6:00 AM. Food and water were provided ad libitum. All experiments were performed in compliance with the guide for the Principles of Laboratory Animal Care (National Institutes of Health publication 85-23, revised 1996).
Head Twitch Response. Mice were transferred from the colony room to the observation room and allowed to habituate for 30 min. All experiments used a between-subject design. Mice (n = 6) were treated one at a time in random order with LY341495 (3.0 mg/kg i.p.) or saline (intraperitoneally) followed 10 min later with either BINA (65 mg/kg i.p.) or vehicle and 30 min later with (-)DOB (0.3 mg/kg s.c.). Immediately after injection of (-)DOB, mice were placed in a 3 L glass beaker and observed for 30 min by two observers, one of whom was blinded to the treatment. Head twitch responses were recorded in 5-min time bins. Counts from the two observers were averaged, with data shown to be consistent between the two. Three or four mice were evaluated per day, between 1:00 and 4:00 PM, and treatments were randomized across the test days.
Fos Expression Experiments. Mice were handled daily for 2 weeks before drug treatment. On the day of treatment, mice were moved from the colony room into an isolation room and allowed to habituate for 30 min. Mice (n = 7) were given either LY341495 (3.0 mg/kg i.p.) or saline followed 10 min later by BINA (65 mg/kg i.p.) or vehicle and 30 min later with (-)DOB (1.0 mg/kg s.c.) or saline (subcutaneously). Experiments were performed between 10:30 AM and 4:30 PM over 3 consecutive days with 12 to 20 animals evaluated per day. Treatments were randomized throughout the 3 days.
The immunohistochemical procedure was modified from a study published previously performed in rats (Gresch et al., 2002). Mice were deeply anesthetized with pentobarbital (250 mg/kg) and transcardially perfused with 0.1 M phosphate-buffered saline and then with 0.1 M phosphate-buffered saline containing 4% paraformaldehyde. Brains were fixed overnight in paraformaldehyde and stored in a solution of 30% sucrose until sectioned. Coronal sections (40 μm) were made using a sliding microtome and placed in cryoprotectant solution (30% glycerol, 30% ethylene glycol, 30% Milli-Q water, and 10% 0.2 M phosphate buffer). Immunohistochemistry was performed on free-floating sections. Slices were treated with 0.3% hydrogen peroxide for 20 min and blocking solution (5% normal goat serum, 2% Triton X-100) for 1 h, Fos primary antibody (Ab-5, polyclonal anti-sera; Calbiochem, La Jolla, CA) diluted 1:20,000 in blocking solution for 48 h at 4°C, secondary antibody (biotinylated anti-rabbit IgG; Vector Laboratories, Burlingame, CA), diluted 1:1000 in blocking solution for 90 min, and finally ABC solution (Vector Laboratories) for 60 min. For colorimetric detection of Fos-like immunoreactivity (Fos-LI), washed sections were treated for 7 min with 3,3′-diaminobenzidine in 0.001% hydrogen peroxide.
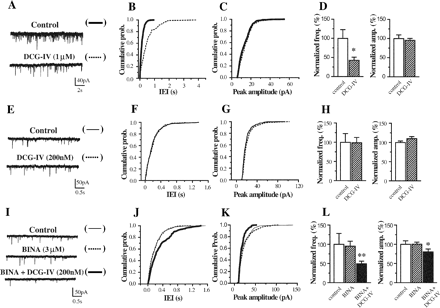
Synergistic action of DCG-IV and BINA on baseline spontaneous EPSCs. Cumulative probability plots for the cell illustrated in A shows the effect of the mGlu2/3 receptor agonist DCG-IV (1 μM) on the frequency (B) and amplitude (C) of baseline EPSCs measured in layer V pyramidal neurons of the rat mPFC. Averaged (n = 5) frequency and amplitude of baseline EPSCs are presented in D. Data are normalized to control condition. Significant difference from control indicated by asterisk (*, p < 0.05, Student's t test). Cumulative probability plots for the cell illustrated in E shows the effect of DCG-IV (200 nM) on the frequency (F) and amplitude (G) of baseline EPSCs. Averaged (n = 6) frequency and amplitude are presented in H. Cumulative probability plots for the cell illustrated in I shows the effect of the mGlu2 receptor allosteric potentiator BINA (3 μM) alone (n = 6) and in the presence (n = 6) of DCG-IV (200 nM) on the frequency (J) and amplitude (K) of baseline EPSCs with L showing averaged frequency and amplitude. Asterisks indicate significant differences from control (*, p < 0.05; **, p < 0.01; Student's t test).
Fos-LI-positive cells were counted in brain sections containing mPFC and somatosensory cortex (SSC) (Franklin and Paxinos, 2003). For each subject, two slices were analyzed bilaterally, resulting in four replicate determinations. Bright-field video images were captured using Openlab 2.2.5 software (Improvision, Lexington, MA) with a Coolsnap cf (Photometrics, Tuscon, AZ) video camera system mounted on a Ziess Axiovert S100 microscope at 10× magnification (Carl Zeiss, Thornwood, NY). The number of Fos-LI-positive cells in a 600 × 450 μm area was counted using ImageJ 1.33u (Wayne Rasband, National Institutes of Health, Bethesda, MD) software. Two program parameters, pixel density and size, were used to define the threshold for a positive count. The four replicate determinants were averaged for each subject.
Drugs
BINA was synthesized by the Vanderbilt Institute for Biological Chemistry Core, as described previously (Galici et al., 2006). For in vitro experiments, BINA was dissolved in dimethyl sulfoxide and then diluted to the appropriate concentration. For in vivo experiments, BINA was dissolved in 10% Tween 80 and 10% 1 N NaOH, and the pH was adjusted to 7.4 using 8.5% lactic acid. (-)DOB was provided by the National Institute on Drug Abuse (Bethesda, MD). LY341495 and DCG-IV [(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl-)glycine] (Tocris Bioscience, Ellisville, MO) were dissolved in deionized water. M100907, a gift from Merrill Dow (Cincinnati, OH), was dissolved in a minimal volume of 2% tartaric acid and brought up to volume in deionized water. LY379268, a gift from Eli Lily and Co. (Indianapolis, IN), was dissolved in deionized water.
Statistical Analyses
In electrophysiological experiments, EPSCs were detected and analyzed using Mini Analysis Program (Synaptosoft, Decatur, GA). The peak amplitude and interevent interval of EPSCs from 30-s episodes during control and drug application were used to generate cumulative probability plots, and the statistical significance was determined by Kolmogorov-Smirnov test. The mean values of EPSC amplitude and interevent interval from the 30-s episode were grouped (mean ± S.E.M.) and compared using a paired two-tail t test; p value <0.05 was considered statistically significant.
For head twitch and Fos expression studies, data were compiled and analyzed using one-way ANOVA, with Newman-Keuls post hoc tests (GraphPad Prism 4.00; GraphPad Software Inc., San Diego, CA). Statistical difference was defined as p < 0.05. Head twitch and Fos expression data are expressed as mean ± S.E.M.
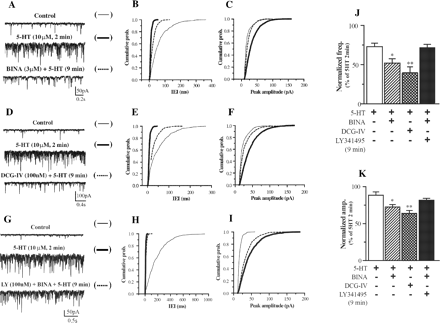
Attenuation of 5-HT-induced EPSCs by BINA and DCG-IV and the reversal of BINA by LY341495. The effect of BINA (3 μM) on 5-HT (10 μM)-induced EPSCs is shown in A to C. Cumulative probability plots for the traces of the cell illustrated in A show a reduction in frequency (B) and amplitude (C) of 5-HT-induced EPSCs. The effect of DCG-IV (100 nM) on 5-HT-induced EPSCs is illustrated in D to F. Cumulative probability plots for the traces of the cell illustrated in D show a reduction in frequency (E) and amplitude (F) of 5-HT-induced EPSCs. LY341495 (100 nM) prevents the effect of BINA on 5-HT-induced EPSCs (G-I). Cumulative probability plots for the traces of the cell illustrated in G show a minimal change in frequency (H) and amplitude (I) of 5-HT-induced EPSCs. Average frequency (J) and amplitude (K) of 5-HT (10 μM) induced EPSCs after 9 min of stimulation in the presence of BINA (3 μM), DCG-IV (100 nM), BINA (3 μM), and LY341495 (100 nM) or control were normalized to maximum 5-HT stimulation observed at 2 min (n = 5-6). Asterisks indicate significant difference from control (*, p < 0.05; **, p < 0.01; Student's t test).
Results
BINA Acts Synergistically with mGlu2/3 Receptor Agonist to Block Baseline Spontaneous EPSCs. Consistent with previous studies, 1 μM DCG-IV, mGlu2/3 receptor agonist, reduces the frequency of spontaneous EPSCs in layer V pyramidal neurons in the mPFC (Fig. 1, A-D), whereas 200 nM DCG-IV was ineffective (Fig. 1, E-H). Furthermore, a concentration of BINA (3 μM) that maximally potentiates mGlu2 receptor-mediated responses in cell lines (Galici et al., 2006) had no effect on spontaneous EPSCs (Fig. 1, I-L). However, the addition of 200 nM DCG-IV in the presence of 3 μM BINA reduced both the frequency and amplitude of the EPSCs to 49.7 ± 6.3 and 80.6 ± 7.0% of control values, respectively (Fig. 1, I-L). This synergistic interaction of BINA and DCG-IV on baseline EPSCs demonstrates that BINA acts as an allosteric potentiator of the mGlu2 receptor.
BINA Attenuates 5-HT-Induced EPSCs, Mimicking mGlu2/3 Receptor Agonist, and Is Blocked by an mGlu2/3 Receptor Antagonist. Application of 5-HT (10 μM) induced a robust increase in the frequency and amplitude of spontaneous EPSCs, which was maximal at 2 min and diminished after 9 min of persistent activation with frequency and amplitude equal to 73.1 ± 4.2 and 88.6 ± 4.3% of the stimulation measured at 2 min (data not shown). The 9-min time point is used for further drug treatment comparison, with data normalized to the maximal stimulation observed at 2 min. The 5-HT-induced EPSCs were blocked by the 5-HT2A receptor antagonist M100907 (100 nM) and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist 6-cyano-2,3-dihydroxy-7-nitroquinoxaline (20 μM) (data not shown), consistent with the interpretation that 5-HT2A receptor activation modulates excitatory signaling via release of glutamate.
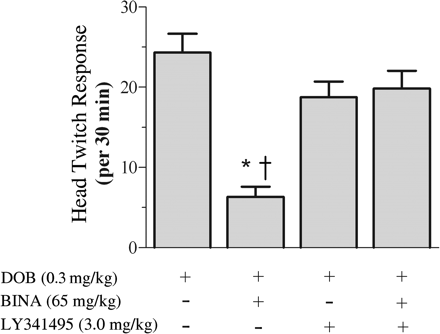
LY341495 reverses BINA-mediated blockade of (-)DOB-induced head twitch response. All mice were administered 0.3 mg/kg (-)DOB (subcutaneously). Animals were divided into four pretreatment groups (n = 6), given saline or LY341495 (3.0 mg/kg i.p.) 10 min before vehicle, or given BINA (65 mg/kg i.p.) 30 min before (-)DOB. *, saline/BINA versus saline/vehicle (p < 0.001, Newman-Keuls). †, saline/BINA versus LY341495/BINA (p < 0.001, Newman-Keuls). LY341495/vehicle pretreatment is not different from saline/vehicle or LY341495/BINA.
The 5-HT-induced increase in spontaneous EPSCs is regulated by mGlu2/3 receptors, acting as presynaptic autoreceptors on thalamocortical glutamatergic terminals within the mPFC (Marek et al., 2000). Although not directly addressed, the current data are consistent with the interpretation that a similar mechanism explains the action of the mGlu2/3 receptor agonists in our preparation. Comparison of 5-HT-induced EPSCs in the presence of BINA (3 μM) and DCG-IV (100 nM) demonstrates the ability of selective mGlu2 receptor manipulation to reduce 5-HT-induced EP-SCs in a manner similar to the mGlu2/3 receptor agonist (Fig. 2, A-F). Both frequency (Fig. 2J) and amplitude (Fig. 2K) of 5-HT-induced EPSCs are decreased in the presence of BINA or DCG-IV. BINA significantly reduced the frequency (52.2 ± 5.2% of maximum stimulation) and amplitude (72.7 ± 3.7% of maximum stimulation) of EPSCs, mimicking the effect of DCG-IV (39.6 ± 7.2 and 64.3 ± 3.7%). These values were all significantly lower than those for 5-HT alone. Figure 2, G-I, illustrates that, in the presence of mGlu2/3 receptor antagonist LY341495 (100 nM), BINA (3 μM) failed to reduce the frequency (Fig. 2J) and amplitude (Fig. 2K) of 5-HT-induced EPSCs. These parameters measured in the presence of BINA and LY341495 are similar to values for 5-HT alone.
BINA-Mediated Blockade of (-)DOB-Induced Head Twitch Response Is Reversed by mGlu2/3 Receptor Antagonist. Hallucinogenic drugs have been repeatedly shown to induce a stereotyped head twitch in rats and mice that is linked to activation of 5-HT2A receptors (Schreiber et al., 1995; Willins and Meltzer, 1997; Gewirtz and Marek, 2000; Klodzinska et al., 2002). This stereotypical behavior is robust and distinct from other stereotypical movements, such as rearing, ear scratching, and sniffing. The head twitch response was assessed in male ICR(CD-1) mice; it became apparent within 5 min of subcutaneous injection of (-)DOB (0.3 mg/kg) and peaked approximately 20 min after treatment (data not shown). In agreement with previous studies (Klodzinska et al., 2002), pretreatment with the mGlu2/3 receptor agonist LY379268 (3.0 mg/kg i.p.) significantly reduced the (-)DOB-induced head twitches (data not shown). The ability of BINA (65 mg/kg) to mimic the action of the mGlu2/3 receptor agonist was examined in the presence or absence of the mGlu2/3 receptor antagonist LY341495 to further test the in vivo selectivity of BINA (Fig. 3). One-way ANOVA revealed a significant effect of pretreatment on the (-)DOB-induced head twitch response [F(3,20) = 15.08, p < 0.001]. Post hoc analysis demonstrates that BINA was able to significantly attenuate the (-)DOB-induced head twitch response (6.3 ± 1.2 versus 24.3 ± 2.3). Pretreatment with LY341495 (3.0 mg/kg i.p.) had no effect alone but significantly reversed of the effects of BINA on the (-)DOB-induced head twitch response (19.8 ± 2.0 versus 6.3 ± 1.2).
The in vitro pharmacological profile of BINA for mGlu receptors was extensively evaluated by Galici et al. (2006), showing in cell expression systems that BINA possessed selective activity at mGlu2 receptor to the exclusion of any effect on glutamate-induced signaling at mGlu1, mGlu3, mGlu4, or mGlu5 receptors. Although indicating selectivity of BINA for various mGlu receptor subtypes, the possibility remained that this novel compound interacts with 5-HT2A receptors, which would compromise the interpretation of its effects in vivo. In control studies, we found that BINA did not alter the binding of the 5-HT2A receptor antagonist [3H]ketanserin or (-)DOB-induced phosphoinositide hydrolysis in cells expressing the 5-HT2A receptor (data not shown), ruling out the possibility that the observed effects of BINA involve direct binding to or allosteric modulation of the 5-HT2A receptor.
BINA Suppresses (-)DOB-Induced Fos Expression in Medial Prefrontal Cortex but Not Somatosensory Cortex. The rapid, transient expression of the immediate early gene c-fos occurs after neuronal activation, thus providing an in vivo map of the cellular response to an excitatory stimulus (Chaudhuri 1997). The protein product of the c-fos gene, Fos, was examined in the mPFC and SSC (Fig. 4). A preliminary dose-response experiment showed that a dose of 1.0 mg/kg (-)DOB resulted in a significant but submaximal Fos signal (data not shown). Pretreatment with M100907 (0.5 mg/kg), a 5-HT2A receptor antagonist, prevented (-)DOB-induced Fos expression in both mPFC and SCC (data not shown). The effects of BINA on the (-)DOB Fos signal in mPFC are summarized in Table 1. One-way ANOVA indicated a significant effect of treatment condition [F(6,42) = 7.12, p < 0.001]; post hoc analysis demonstrated that (-)DOB significantly increased the number of Fos-LI-positive cells compared with the baseline saline treatment. In addition, BINA (65 mg/kg) completely prevented the (-)DOB-induced Fos expression with no effect on baseline Fos expression. Finally, pretreatment with LY341495 (3.0 mg/kg) was able to antagonize the actions of BINA with no significant potentiation of (-)DOB alone. LY341495 induced a significant increase in baseline Fos-LI-positive cells.
Modulation of (−)DOB-induced Fos-like immunoreactivity in medial prefrontal cortex and somatosensory cortex
Animals (n = 6-8 per group) were given LY341495 (3.0 mg/kg i.p.) or saline 10 min before BINA (65 mg/kg i.p.) or vehicle 40 min before (−)DOB (1.0 mg/kg s.c.) or saline. Shown is the number of Fos-LI-positive cells in the region of the mPFC or SSC analyzed. BINA attenuated the (−)DOB-induced Fos expression in the mPFC but not the SSC, an effect reversed by the mGlu2/3 receptor antagonist LY341495. BINA did not significantly alter Fos expression alone. LY341495 significantly increased saline-induced but not (−)DOB-induced Fos expression.
Previous work demonstrated that the hallucinogen-induced c-fos expression was regulated by mGlu2/3 receptors in the mPFC but not in the SSC (Zhai et al., 2003). To determine whether this regional specificity also applies to an mGlu2-selective agent, we extended the Fos analyses to the SSC (Table 1), where a significant effect of treatment condition was found [one-way ANOVA, F(6,42) = 7.12, p < 0.001]. (-)DOB induced a significant increase in Fos-LI-positive cells, with a distribution of Fos-LI-positive cells throughout a “band” from deep layer III through superficial layer V. However, the (-)DOB-induced expression in the SSC was not significantly altered by either BINA or LY341495, demonstrating the specificity of this neuronal response.
Schematic illustrations of the areas in which Fos expression was analyzed. Fos-LI-positive cells were counted in highlighted regions of sections (based on Franklin and Paxinos, 2003) containing medial prefrontal cortex (+1.70 mm relative to bregma) and somatosensory cortex (+0.74 mm relative to bregma).
Discussion
As mGlu2/3 receptor agonists continue to gain notoriety for their antipsychotic actions in humans, reducing symptoms of schizophrenia and the effects of the psychotomimetic ketamine (Krystal et al., 2004; Schoepp, 2006), it is becoming increasingly important to further delineate the individual roles of mGlu2 and mGlu3 receptors. The recent development of BINA, a selective mGlu2 receptor allosteric potentiator, makes possible the pharmacological evaluation of mGlu2 receptors in the absence of any potential confounding activation of mGlu3 receptors. In in vitro electrophysiological and in vivo behavioral and gene expression studies, we show that selective activation of mGlu2 receptors mimics a nonselective mGlu2/3 receptor agonist, attenuating the actions of hallucinogenic drugs and glutamatergic neurotransmission in the mPFC.
Using whole-cell recordings in brain slices, we evaluated the consequences of selective modulation of mGlu2 receptors with the positive allosteric modulator BINA. BINA, at a concentration that had no effect alone, potentiates the actions of a subeffective concentration of DCG-IV to block baseline EPSCs, confirming that BINA acts as an allosteric potentiator of the mGlu2 receptor. At a concentration that had no effect on baseline EPSCs, BINA attenuates 5-HT-induced EPSCs, mimicking the mGlu2/3 receptor agonist DCG-IV. It is noteworthy that BINA is capable of reducing 5-HT-induced EPSCs when added alone, in the absence of exogenous agonist. This is the first example of an electrophysiological response to an allosteric potentiator of a mGlu receptor that does not require concomitant application of an agonist. Because BINA is not capable of directly activating mGlu2 receptors in the absence of an orthosteric agonist (Galici et al., 2006), this suggests that BINA potentiates low levels of endogenous glutamate that have access to presynaptic mGlu2 receptors and that selective activation of mGlu2 receptors is an exquisitely sensitive mechanism for regulation of hyperglutamatergic transmission.
Hallucinogenic drugs have been shown previously to induce glutamatergic signaling in the mPFC, a brain region believed to underlie their behavioral effects and some of the psychological symptoms of schizophrenia. The hallucinogenic 5-HT2A receptor agonist (-)DOB is used here to investigate the role that selective activation of mGlu2 receptors plays in the regulation of glutamatergic neurotransmission. Past studies have provided a hypothetical model of in vivo hallucinogenic drug action (Aghajanian and Marek, 2000) in which agonism at 5-HT2A receptors results in activation of glutamatergic neurotransmission in the mPFC linked to a presynaptic modulation of glutamate release from thalamocortical afferent neuronal terminals (Aghajanian and Marek 1997, 1999; Marek et al., 2000, 2001). Selective expression of mGlu2 receptor mRNA in midline and intralaminar thalamic nuclei (Ohishi et al., 1993a), the region containing neurons known to project to the mPFC, suggests a dominant role of mGlu2 receptor in the presynaptic action of mGlu2/3 receptor ligands at thalamocortical terminals. Our experiments showing that BINA, a selective mGlu2 receptor positive allosteric modulator, attenuates 5-HT-induced EPSCs in the mPFC are consistent with this hypothesis.
A major finding of the current study is the ability of BINA to block the (-)DOB-induced head twitch response, mimicking the actions of an mGlu2/3 receptor agonist, demonstrating that the regulation of hallucinogen-induced behavior is reproduced by the selective activation of mGlu2 receptors. Blockade of head twitch response was observed in a dose range that is similar to the previously reported BINA attenuation of the behavioral effects of the noncompetitive N-methyl-d-aspartate receptor antagonist phencyclidine (Galici et al., 2006). This result suggests that the dampening effects of mGlu2/3 receptor agonists on psychotomimetic drug-induced behaviors (Gewirtz and Marek, 2000; Klodzinska et al., 2002; Winter et al., 2004) are probably caused by the activation of mGlu2 receptor. Furthermore, in vivo selectivity of BINA was confirmed by a reversal of the blockade of head twitch response by pretreatment with the mGlu2/3 receptor antagonist LY341495. These data strongly support a reciprocal relationship between mGlu2 and 5-HT2A receptors in the behavioral actions of hallucinogens and illustrate the significance of mGlu2 receptors as targets for the regulation of behaviors dependent on hyperglutamatergic signaling. LY341495 is only 1 order of magnitude less potent as an antagonist at mGlu8 receptors (Kingston et al., 1998). Because BINA has not been evaluated at this subtype, the possible contribution of mGlu8 receptors, although unlikely, cannot be ruled out.
Delineating the neuroanatomical site of action responsible for the behavioral effects of systemically administered drugs is a difficult task. Microinjection of the hallucinogenic 5-HT2A/2C receptor agonist (±)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane into the mPFC of rats has been shown to induce the head twitch response (Willins and Meltzer, 1997). Here, we confirm this site using an additional design often used to link the behavioral actions of drugs to a brain site of action, in vivo induction of immediate early gene expression. The induction of the immediate early gene c-fos and the expression of its protein product Fos is a postsynaptic event linked to the activation of ionotropic glutamate receptors (Hughes and Dragunow, 1995; Platenik et al., 2000). (-)DOB induced Fos expression in mPFC, anterior cingulate cortex, frontoparietal cortex, and SSC in a pattern similar to that observed previously with other hallucinogens (Leslie et al., 1993; Abi-Saab et al., 1998; Scruggs et al., 2000; Gresch et al., 2002; Zhai et al., 2003). In the current experiments, we used a drug treatment regimen to regulate Fos expression that closely mimicked the one shown to be behaviorally effective. At a behaviorally active dose, BINA attenuated (-)DOB activation of Fos expression in the mPFC but not in the SSC, mimicking the effect of the mGlu2/3 receptor agonist LY379268 (Zhai et al., 2003). Although both of these regions have been shown to display 5-HT2A receptor-mediated Fos expression, only expression in the mPFC is regulated by mGlu2 receptors, a difference linked to distinct mGlu2 receptor expression in the thalamic neurons projecting to these regions (Ohishi et al., 1993a,b). Therefore, the blockade of (-)DOB-induced Fos expression in the mPFC, but not SSC, by the selective activation of mGlu2 receptors further supports the role of glutamate released from thalamocortical afferents in the in vivo actions of hallucinogens. In addition, these observations are consistent with the neuroanatomical specificity of the actions of mGlu2 receptors, illustrating that in vivo mGlu2 receptors are key regulators of glutamatergic neurotransmission in select regions of the cortex, notably the mPFC.
This study takes a comprehensive experimental approach to delineate the role of mGlu2 receptors in the regulation of glutamatergic neurotransmission in the mPFC and in a model of psychosis. Results with the selective mGlu2 receptor allosteric potentiator BINA demonstrate that this novel class of allosteric regulators of metabotropic receptors elicits robust in vitro and in vivo effects. BINA attenuated the 5-HT-mediated EPSCs in layer V pyramidal neurons, confirming a physiological antagonism between mGlu2 receptors and 5-HT2A receptors. Analysis of BINA regulation of (-)DOB-induced Fos expression and head twitch response clearly indicates that selective activation of mGlu2 receptors is capable of attenuating hallucinogenic drug action and illustrates their convergence on glutamatergic neurotransmission in the mPFC. This class of glutamatergic drugs, whose function is mechanistically distinct from the direct agonists, warrants further investigation for their therapeutic potential. Because they interact with an allosteric site to potentiate endogenous glutamate, allosteric potentiators are predicted to produce more subtle, ordered effects and hence be sheltered from potential pitfalls of the direct agonists, such as desensitization and tolerance development.
Acknowledgments
We thank Kathleen Patterson for skillful technical assistance.
Footnotes
- Received February 13, 2007.
- Accepted May 25, 2007.
This research was supported in part by National Institutes of Health research grants MH34007 and DA05181 and by grants from the National Institute of Mental Health (to E.S.-B., P.J.C.), National Institute on Drug Abuse (to E.S.-B.), and National Institute of Neurological Disorders and Stroke (to P.J.C.).
M.A.B. and Z.X. contributed equally to this work.
ABBREVIATIONS: mGlu, metabotropic glutamate; mPFC, medial prefrontal cortex; BINA, biphenyl-indanone A; 5-HT, serotonin; 5-HT2A, serotonin subtype 2A; EPSC, excitatory postsynaptic current; SSC, somatosensory cortex; Fos-LI, Fos-like immunoreactive; ACSF, artificial cerebrospinal fluid; LY341495, 2S-2-amino-2-(1S,2S-2-carboxycyclopropan-1-yl)-3-(xanth-9-yl)propionic acid; (-)DOB, (-)2,5-dimethoxy-4-bromoamphetamine; DCG-IV, (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine; M100907, R-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol; LY379268, (-)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate; ANOVA, analysis of variance.
- The American Society for Pharmacology and Experimental Therapeutics