Abstract
Histamine regulates neurotransmitter release in the central and peripheral nervous systems through H3 presynaptic receptors. The existence of the histamine H3 receptor was demonstrated pharmacologically 15 years ago, yet despite intensive efforts, its molecular identity has remained elusive. As part of a directed effort to discover novel G protein-coupled receptors through homology searching of expressed sequence tag databases, we identified a partial clone (GPCR97) that had significant homology to biogenic amine receptors. The GPCR97 clone was used to probe a human thalamus library, which resulted in the isolation of a full-length clone encoding a putative G protein-coupled receptor. Homology analysis showed the highest similarity to M2 muscarinic acetylcholine receptors and overall low homology to all other biogenic amine receptors. Transfection of GPCR97 into a variety of cell lines conferred an ability to inhibit forskolin-stimulated cAMP formation in response to histamine, but not to acetylcholine or any other biogenic amine. Subsequent analysis revealed a pharmacological profile practically indistinguishable from that for the histamine H3 receptor. In situ hybridization in rat brain revealed high levels of mRNA in all neuronal systems (such as the cerebral cortex, the thalamus, and the caudate nucleus) previously associated with H3 receptor function. Its widespread and abundant neuronal expression in the brain highlights the significance of histamine as a general neurotransmitter modulator. The availability of the human H3 receptor cDNA should greatly aid in the development of chemical and biological reagents, allowing a greater appreciation of the role of histamine in brain function.
Since its first pharmacological description as an endogenous substance in 1910 (Barger and Dale, 1910), histamine has proven to exert tremendous influence over a variety of physiological processes. Most notable are its roles in the inflammatory “triple response” and in gastric acid secretion, which are mediated by H1 (Ash and Schild, 1966) and H2 (Black et al., 1972) receptors, respectively. In the early 1970s emerged an understanding that histamine is a neurotransmitter in the central nervous system (Schwartz et al., 1970;Baudry et al., 1975). In 1983, a third subtype of histamine receptor, H3, was identified as a presynaptic autoreceptor on histamine neurons in the brain controlling the stimulated release of histamine (Arrang et al., 1983). Subsequently, the H3 receptor has been shown to be a presynaptic heteroreceptor in nonhistamine-containing neurons in both the central and peripheral nervous systems (for review, see Hill et al., 1997). Through the molecular cloning of H1 and H2, these receptors were proven to belong to the superfamily of G protein-coupled receptors (GPCRs; Gantz et al., 1991;Yamashita et al., 1991). For the past 10 years, the histamine H3 receptor has been the target of numerous cloning and purification attempts, yet its molecular identity has remained an enigma.
We have initiated an effort to identify and clone orphan GPCRs as a means to identify novel drug targets and as a way to discover novel neurotransmitters and peptides. This is an approach used by many investigators, and it has led to the successful identification of ligands such as nociceptin (Reinscheid et al., 1995), prolactin-releasing factor (Hinuma et al., 1998), the orexins (Sakurai et al., 1998), and, more recently, apelin (Tatemoto et al., 1998). There are at least 70 orphan GPCRs in the public domain. We have identified, through searching public and private databases, at least 30 additional putative members of this family via expressed sequence tags (ESTs). One of these orphan receptors, our designation GPCR97, was expressed abundantly in the central nervous system, and its 5′-most sequence shares significant homology with the putative transmembrane domain VII of several members of the biogenic amine family of receptors. Therefore, we investigated the possibility that the GPCR97 cDNA encodes a novel neurotransmitter receptor.
Experimental Procedures
Materials.
Human mRNA and all Northern blots were purchased from Clontech (Palo Alto, CA). cDNA synthesis kits were purchased from Gibco Life Technologies (Gaithersburg, MD). Gelzyme was obtained from Invitrogen (San Diego, CA), and pCIneo vector was obtained from Promega (Madison, WI). All cell lines were obtained from American Type Culture Collection (Manassas, VA). Cyclic AMP (cAMP) Flashplates were obtained from DuPont/New England Nuclear (Boston, MA). Fluo-3 was purchased from TEF Laboratories (Austin, TX) G418 was purchased from Calbiochem (San Diego, CA). All histamine ligands were purchased from Research Biochemicals, Inc. (Natick, MA). All other reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Cloning of GPCR97 cDNA.
A human thalamus cDNA library was constructed from poly(A)+-selected RNA as described by the manufacturer (Gibco Life Technologies). Double-stranded DNA was digested with NotI and then run on a 0.8% low-melting agarose gel, and cDNA in the range of 2.5 to 5 kilobases (kb) was excised, purified with Gelzyme, and subsequently was subcloned into pSport vector. The size-selected human thalamus cDNA library was screened with a radiolabeled fragment of the GPCR97 EST clone. A full-length GPCR97 was obtained and, subsequently, cloned into the mammalian expression vector pCIneo (Promega) and transfected into human embryonic kidney 293 cells, rat C6 glioma cells, and human SK-N-MC neuroblastoma cells.
Transfection of Cells with GPCR97 cDNA.
Cells were grown to about 70% to 80% confluence and then removed from the plate with trypsin and pelleted in a clinical centrifuge. The pellet was then resuspended in 400 μl of complete media and transferred to an electroporation cuvette with a 0.4-cm gap between the electrodes (no. 165–2088; Bio-Rad Laboratories, Hercules, CA). One microgram of supercoiled DNA was added to the cells and mixed. The voltage for the electroporation was set at 0.25 kV and the capacitance was set at 960 μF. After electroporation, the cells were diluted into 10 ml of complete media and were plated onto four 10-cm dishes at the following ratios: 1:20, 1:10, 1:5, and the remaining cells. The cells were allowed to recover for 24 h before the addition of G-418. Colonies that survived selection were grown and tested. Several different cell lines were used for transfection, which served two purposes. First, because single-cell cloning can often uncover endogenously expressed receptors (unpublished observations), it is imperative to see the desired function in multiple transfections in different cell lines. Second, each cell line has a unique characteristic that can be used to enhance different aspects of the study. For example, C6 cells grow very fast and are easy to culture and, thus, are good for generating lots of membranes for binding. SK-N-MC cells give robust cAMP accumulation and give efficient coupling for inhibition of adenylate cyclase. L cells consistently transfect well and have few endogenous receptors, and, thus, are good for reliable initial characterization of recombinant receptors. It should be noted that inhibition of adenylate cyclase and [3H]R-α-methylhistamine binding were observed in all of the GPCR97-transfected cells. Only the best responding cell lines were used for further study.
cAMP Accumulation.
Transfected cells were plated on 96-well plates. Overnight cultures were then incubated with Dulbecco’s modified Eagle’s medium-F12 media containing isobutylmethylxanthine (2 mM) for 20 min, treated with agonists, antagonists, or both for 5 min, and then treated with forskolin (10 μM) for 20 min. The reaction was stopped with 1/5 volume 0.5 N HCl. Cell media were then tested for cAMP concentration by radioimmunoassay with cAMP Flashplates.
Calcium Mobilization.
Transfected cells were plated on black 96-well plates with clear bottoms. Overnight cultures were then incubated with Dulbecco’s modified Eagle’s medium-F12 media containing the fluorescent calcium indicator fluo-3 (4 μM) and probenicid (2 mM) for 60 min. Ligand-induced fluorescence was then measured on a Fluorometric Imaging Plate Reader (FLIPR; Molecular Devices, Sunnyvale, CA).
R-α-Methyl[3H]histamine Binding.
Cell pellets from GPCR97-expressing C6 cells were homogenized in 20 mM Tris-HCl/0.5 mM EDTA. Supernatants from a 800g spin were collected and recentrifuged at 30,000g for 30 min. Pellets were rehomogenized in 50 mM Tris/5 mM EDTA (pH 7.4). Membranes were incubated with 0.4 nMR-α-methyl[3H]histamine plus/minus test compounds for 45 min at 25°C and harvested by rapid filtration over GF/C glass fiber filters (pretreated with 0.3% polyethylenimine), followed by four washes with ice-cold buffer. Nonspecific binding was defined with 10 μM histamine. pKI values were calculated based on a K d of 150 pM and a ligand concentration of 400 pM (Cheng and Prusoff, 1973).
In Situ Hybridization.
Three adult male Sprague-Dawley rats were perfused with 4% paraformaldehyde in 0.1 M borate buffer fixative, and their brain tissues were postfixed overnight in fixative with 10% sucrose and frozen in dry ice. Five 1-in-5 series of 30-μm-thick coronal sections of the whole brain were cut on a sliding microtome and mounted onto glass slides. In situ hybridization was performed with 35S-riboprobes on this tissue by an adapted protocol (Simmons et al., 1989). Then the tissue samples were put on X-ray film for 1 day, after which they were dipped in NBT2 nuclear emulsion (Eastman Kodak Co., Rochester, NY), and kept desiccated in the dark at 4°C for 6 days. Slides were developed, were Nissl stained, and were studied under the microscope to identify structures labeled with the GPCR97 cRNA probe.
RNA Probes.
The cRNA probe was constructed from a partial rat GPCR97 cDNA clone originally identified by polymerase chain reaction (PCR) amplification from rat brain cDNA with primers designed against the human receptor (5′ primer, 5′-AGTCGGATCCAGCTACGACCGCTTCCTGTC-3′; 3′ primer, 5′-AGTCAAGCTTGGAGCCCCTCTTGAGTGAGC-3′). The resulting 607-base pair (bp) fragment was ligated into pBluescript (Stratagene, La Jolla, CA).35S-UTP-labeled antisense and sense probes for rat GPCR97 were synthesized after linearization with BamHI or HindIII with T7 or T3 RNA polymerase, respectively. The labeled sense strands served as controls and did not show any specific labeling of cellular localization (data not shown). Specific activities of 35S-UTP probes were approximately 2 to 3 × 106 counts per minute/μg. All restriction enzymes and phage RNA polymerases were obtained from Boehringer Mannheim (Indianapolis, IN).
Northern Blot Analysis.
Northern blots obtained from Clontech (Palo Alto, CA) were hybridized with α-32P-dCTP-labeled (Amersham Pharmacia Biotech, Piscataway, NJ) human GPCR97 cDNA as described by the manufacturer (Expresshyb, Clontech). Two million counts per milliliter was used in a total volume of 10 ml of hybridization buffer and incubated at 68°C for 2 h. The blot was then washed two times at RT in 2 × standard saline citrate and 0.05% SDS for 30 min each. It was further washed two more times for 30 min each at 60°C and exposed overnight to film.
Results
Cloning and Sequence Analysis of GPCR97 cDNA.
GPCR97 was initially identified as an EST in a basic local alignment search tool (Altschul et al., 1990) search of the Life Seq database (Incyte Pharmaceuticals, Palo Alto, CA) with the α2-adrenergic receptor sequence as a query. The 5′ end of the GPCR97 EST had approximately 35% homology to the seventh transmembrane domain of the α2-adrenergic receptor. Semiquantitative PCR of GPCR97 with cDNA templates from a variety of human tissues showed expression predominantly in the central nervous system, with the greatest intensity in the thalamus. Therefore, we constructed a size-selected human thalamus cDNA library and screened it with the original EST fragment as a labeled probe. From this screen, a full-length 2.7-kb clone consisting of a 298-bp 5′-untranslated region, a 1335-bp open reading frame, and a 1100-bp 3′-untranslated region was obtained. Translation of the open reading frame revealed a 445-amino acid coding region with low homology (20–27%) to the biogenic amine subfamily of GPCRs. Most notable was an aspartic acid residue in the putative transmembrane domain III, the putative binding site for the primary amine, which is a clear hallmark of the biogenic amine receptor subfamily (Fig. 1). This conserved aspartic acid residue is shown in the alignment of the predicted amino acid sequence of GPCR97 with the human histamine H1 and H2 receptors. Overall homology between GPCR97 and the H1 and H2 receptors is 22% and 21.4%, respectively.
Amino acid sequence of human GPCR97 receptor compared with the human histamine H1 and H2 receptors. Putative transmembrane domains are stated above the sequence and indicated by a solid line. Residues that are identical among all three receptors are indicated by an ∗ below the sequence. DNA and protein sequences have been deposited with GenBank (accession no. AF140538)
GPCR97-Expressing Cells Inhibit Adenylate Cyclase in Response to Histamine.
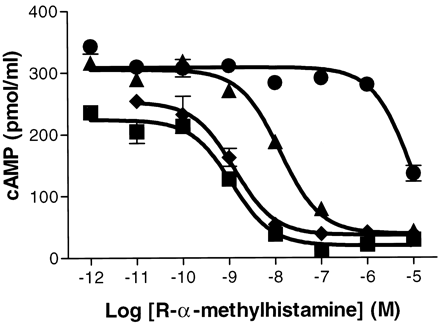
Given the homology of GPCR97 to the biogenic amine family, we first tested its ability to respond to several of the amine neurotransmitters, measuring either the stimulation of calcium mobilization or the increase or decrease of cAMP accumulation in mouse L cells. The biogenic amine ligands tested (acetylcholine, dopamine, imidazole, epinephrine, tryptamine, serotonin, and histamine) were negative for an increase in both calcium mobilization or in cAMP accumulation (not shown). However, after forskolin stimulation of basal cAMP accumulation, there was a selective and marked inhibition of adenyate cyclase in response to histamine in the transfected cell line but not in the nontransfected cell line (Fig.2). This effect was mimicked by the high-affinity H3 agonistR-α-methylhistamine, which has an EC50 of 1 nM (Fig.3). In addition, the effect ofR-α-methylhistamine could be blocked by the known selective H3 antagonists thioperamide and clobenpropit (Fig. 3) but not by the H1antagonist diphenhydramine (Fig. 3) or the H2antagonist ranitidine (not shown).
Inhibition of cAMP accumulation in response to the various amine transmitters. Cells were treated with 10 μM forskolin 5 min after the addition of compounds (1 μM) and incubated for an additional 20 min. All values were determined in duplicate. Error bars represent S.E.M.
Inhibition of cAMP accumulation in response to the agonist R-α-methylhistamine. Cells were treated with 10 μM forskolin 5 min after the addition ofR-α-methylhistamine and incubated for an additional 20 min. Where indicated, antagonists (1 μM) were incubated 5 min before the addition of the agonist alone (▪), with diphenhydramine (♦), with thioperamide (▴), or with clobenpropit (●). All values are determined in triplicate. Error bars represent S.E.M.
GPCR97-Expressing Cells Bind the High-Affinity Histamine H3 LigandR-α-Methyl[3H]histamine.
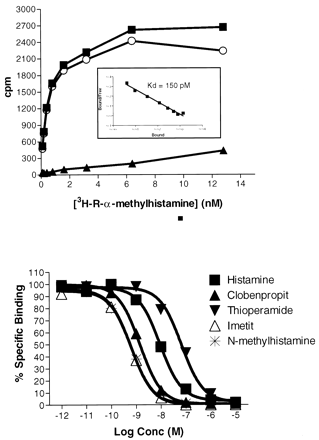
To confirm the H3 pharmacology, we examined whether the GPCR97-transfected cells could bind the H3 ligandR-α-methyl[3H]histamine. For these studies, we transfected a different cell line (C6 glioma cells) because of its of ability to grow fast. C6 cells transfected with GPCR97 were able to bind [3H]R-α-methylhistamine with high affinity (Fig. 4, inset), whereas untransfected cells had no demonstrable binding (not shown). In addition, the known H3 agonists (histamine, imetit, and N-methylhistame) and antagonists (thioperamide and clobenpropit) could all compete for binding (Fig. 4) with a rank order of potency consistent with that described for the histamine H3 receptor (Table1).
Top, saturation isotherm and Scatchard transformation (inset) of R-α-methyl[3H]histamine to GPCR97-transfected C6 cells. Total binding (▪), nonspecific binding (▴), and specific binding (○) are shown. Bottom, competition binding of [3H]R-α-methylhistamine (0.4 nM) in the presence of various concentrations of H3agonists and antagonists. K D was calculated as −1/slope from the linear Scatchard transformation. pIC50 values were determined by a single site curve fitting program (Prism; GraphPad Software, San Diego, CA) and converted to pKI values according to Cheng and Prusoff (1973).
pKI values of known histamine agonists and antagonists
GPCR97 is Expressed Abundantly in the Central Nervous System.
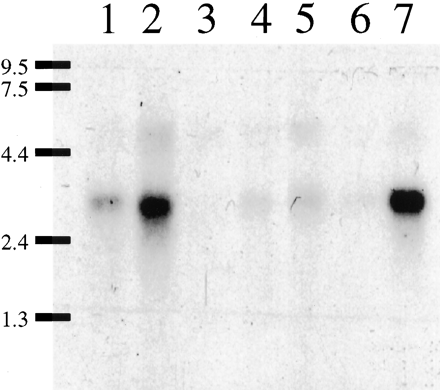
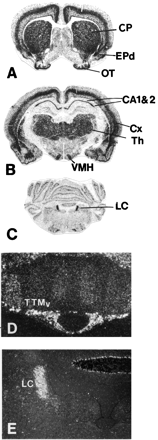
Because the pharmacological profile of GPCR97 was consistent with the histamine H3 receptor, we investigated the mRNA distribution and compared it to the known distribution of H3 binding sites. Northern blots of human mRNA showed expression only in the brain, most notably in the thalamus and the caudate nucleus (Fig. 5). Little expression was observed in any peripheral tissue examined (heart, placenta, lung, liver, skeletal muscle, kidney, pancreas, spleen, thymus, prostate, testis, ovaries, small intestine, colon, stomach, thyroid, lymph node, trachea, and bone marrow; data not shown). To obtain a rat homolog of the GPCR97 cDNA, we used oligonucleotide primers designed from the human sequence to amplify a cDNA fragment from RNA extracted from rat brain. This rat cDNA probe (which has 85% nucleotide identity to human GPCR97) was subsequently used to examine the tissue distribution of GPCR97-encoded mRNA by in situ hybridization in rat brain sections. GPCR97 mRNA is abundantly expressed in rat brain and is most notably observed throughout the thalamus, the ventromedial hypothalamus, and the caudate nucleus (Fig.6, A and B). Strong expression was also seen in layers II, V, and VIb of the cerebral cortex, in the pyramidal layers (CA1 and CA2) of the hippocampus, and in olfactory tubercle (Fig. 6, A and B). Because the H3receptor functions as an inhibitory presynaptic receptor, it is expected that the mRNA localization may not exactly match the functional receptor localization, depending on the axonal length of the neuron expressing it. For example, noradrenergic cells in the locus ceruleus project to all areas of the cerebral cortex where histamine, via H3 receptors, is known to regulate noradrenaline release (Schlicker et al., 1989; Smits and Mulder, 1991). Therefore, it was predicted and confirmed that the mRNA for GPCR97 was expressed in the locus ceruleus (Fig. 6, C and E). In addition, because the H3 receptor has also been functionally demonstrated on the histamine terminals in the cerebral cortex (Arrang et al., 1983), its mRNA must also be located in the histaminergic cell bodies in the tuberomammillary nuclei. This was also confirmed for GPCR97 (Fig. 6D).
Northern blot analysis of human brain mRNA samples (5 ug of poly(A)+ RNA/lane). Lane 1, amygdala. Lane 2, caudate. Lane 3, corpus callosum; Lane 4, hippocampus. Lane 5, whole brain. Lane 6, substantia nigra. Lane 7, thalamus. The probe was the full-length GPCR97 coding sequence. Exposure time to film was 3 days (−80°C).
Distribution of GPCR97 mRNA in rat brain. Representative film autoradiograms of coronal sections arranged rostral to caudal (A-C) and darkfield photomicrographs of coronal brain sections showing GPCR97 mRNA in the ventral portion of the tuberomammillary nucleus (D), and in the locus ceruleus (E). Magnification, D = 100× and E = 40×. Abbreviations: CA1, CA2, pyramidal layers of the hippocampus; CP, caudoputamen; Cx, cortex; EPd, endopiriform nucleus, dorsal part; LC, locus ceruleus; OT, olfactory tubercle; Th, thalamus; TTMv, tuberomammillary nucleus, ventral portion; VMH, ventromedial hypothalamus.
There are numerous reports of presynaptic H3receptors in the autonomic nervous system controlling neurotransmitter release in the heart, the lung, and the gastrointestinal tract (Arrang et al., 1988; Molderings et al., 1992; Bertaccini and Coruzzi, 1995;Imamura et al., 1995; Stark et al., 1996a). GPCR97 mRNA was detected by PCR amplification in RNA extracted from human small intestine, testis, and prostate tissues, but was not detected in these tissues by Northern blot analysis (not shown). If GPCR97 was only expressed in the neuronal plexus, its overall low abundance in a whole tissue preparation could account for this discrepancy. We are currently investigating via in situ hybridization whether the GPCR97 receptor mRNA is produced in the ganglia of the autonomic and enteric nervous systems. An alternative explanation for the absence of clear peripheral expression could be the existence of additional subtypes of the H3 receptor, which previously has been suggested based on pharmacological evidence (West et al., 1990; Raible et al., 1994; Leurs et al., 1996; Schlicker et al., 1996).
Discussion
The present data describes the cloning and characterization of a novel GPCR, GPCR97, with a pharmacology and a tissue distribution that is consistent with the histamine H3 receptor subtype. We found that cells transfected with GPCR97 were able to inhibit adenylate cyclase in response to histamine. Because the two known cloned histamine receptors, H1 and H2, activate phosphoinositide hydrolysis and stimulation of adenylate cyclase, respectively, the inhibition of adenylate cyclase that we observed is a new finding for a cloned histamine receptor. It should be noted that previous experiments with pertussis toxin- and histamine-stimulated35S-GTPγS binding have suggested that the H3 receptor might be Gi-linked (Clark et al., 1993; Laitinen and Jokinen, 1998). Because the putative H3 histamine receptor has been pharmacologically defined (Arrang et al., 1987; Leurs et al., 1998), we were able to test known selective agonists and antagonists. The selective H3 agonistR-α-methylhistamine was able to potently and dose-dependently inhibit forskolin-stimulated adenylate cyclase, an effect that was mimicked by two additional H3agonists, imitet and N-α-methylhistamine (data not shown). In addition, the effect of R-α-methylhistamine was blocked by the selective H3 antagonists thioperamide and clobenpropit but not by the H1 or H2 antagonists diphenhydramine or ranitidine. GPCR97-transfected cells also bound the high-affinity H3 agonistR-α-methyl[3H]histamine. All of the tested H3 agonists and antagonist could compete for specificR-α-methyl[3H]histamine binding with similar potencies to those reported for these compounds to brain membranes (Hill et al., 1997). It has been suggested that clozapine may impart some of its antipsychotic effects in humans through H3 receptor antagonism (Kathmann et al., 1994;Rodrigues et al., 1995; Stark et al., 1996b). We found that clozapine did not significantly compete for binding to the recombinant human receptor (Table 1). These differences in pharmacology may be because of species differences or possible H3heterogeneity (West et al., 1990).
One of the most striking features of this receptor is the abundant expression in the central nervous system, particularly in the caudate, the thalamus, and the cortex. Thus, it is surprising that this receptor cDNA has eluded so many cloning attempts over the years. To explain the previous unsuccessful attempts to clone the H3receptor, we compared the sequence of GPCR97 to that of the H1 and H2 receptors (Fig.1). The low overall homology among these three receptors suggests, in retrospect, that low-stringency hybridization approaches or degenerate PCR would not have been fruitful. In addition, we searched the public EST databases with the entire H3 receptor mRNA sequence. We found that the H3 receptor exists in the public domain in several clones derived from human brain libraries. However, all of these clones primarily contain only a 3′-untranslated sequence, suggesting that there may be some secondary structure present that prevents a full-length H3 encoding mRNA from being efficiently copied by reverse transcription. Our success in screening the human thalamus may be due to its abundance in that specific brain region, coupled with the fact that we size-selected for mRNAs greater than 2.5 kb.
There are many questions that remain to be answered about the histamine H3 receptor that we can now begin to answer with the cDNA. For example, are there additional H3receptor subtypes? What additional neurotransmitter systems are regulated by histamine H3 receptors? Are H3 receptors expressed on nonneuronal cells in the periphery? We are currently seeking to answer some of these questions. In addition, we are inactivating the H3 receptor gene in mice (i.e., knockout mice) to identify its role in central nervous system function and memory control and as a means to look for additional phenotypes, which may lead to a better understanding of the physiological role of H3 receptors in normal and pathological states.
Acknowledgments
We thank Jose Galindo for his great help in assembling the sequence information and K.C. Joy for performing reverse transcription-PCR experiments. We also thank Drs. Lars Karlsson, Nigel Shankley, and Josee Leysen for providing insightful discussion.
Footnotes
- Received February 12, 1999.
- Accepted April 2, 1999.
-
Send reprint requests to: Dr. Timothy W. Lovenberg, R.W. Johnson Pharmaceutical Research Institute, 3535 General Atomics Ct., San Diego, CA. E-mail:tlovenbe{at}prius.jnj.com
Abbreviations
- GPCR
- G protein-coupled receptor
- EST
- expressed sequence tag
- cAMP
- cyclic AMP
- PCR
- polymerase chain reaction
- The American Society for Pharmacology and Experimental Therapeutics