Abstract
Transmembrane domain 6 of the muscarinic acetylcholine (ACh) receptors is important in ligand binding and in the conformational transitions of the receptor but the roles of individual residues are poorly understood. We have carried out a systematic alanine-scanning mutagenesis study on residues Tyr381 to Val387 within the binding domain of the M1 muscarinic ACh receptor. The seven mutations were then analyzed to define the effects on receptor expression, agonist and antagonist binding, and signaling efficacy. Tyr381Ala produced a 40-fold reduction in ACh affinity and a 50-fold reduction in ACh-signaling efficacy. Leu386Ala had similar but smaller effects. Asn382Ala caused the largest inhibition of antagonist binding. The roles of the hydroxyl group and benzene ring of Tyr381 were probed further by comparative analysis of the Tyr381Phe and Tyr381Ala mutants using three series of ligands: ACh analogs, azanorbornane- and quinuclidine-based ligands, and atropine analogs. These data suggested that the hydroxyl group of Tyr381 is primarily involved in forming hydrogen bond interactions with the oxygen atoms present in the side chain of ACh. We propose that this interaction is established in the ground state and preserved in the activated state of the receptor. In contrast, the Tyr381 benzene ring may form a cation-π interaction with the positively charged head group of ACh that contributes to the activated state of the receptor but not the ground state. However, the hydroxyl group and benzene ring of Tyr381 both participate in interactions with azanorbornane- and quinuclidine-based ligands and atropine analogs in the ground state as well as the activated state of the receptor.
Transmembrane domain (TM) 6 of receptors in the rhodopsin-like family of G protein-coupled receptors has been shown to play a major role both in ligand binding and in producing the resulting functional response (for a recent review see Gether and Kobilka, 1998). There are no data for the M1 muscarinic acetylcholine receptor (mAChR) itself, but in the rat M3 mAChR, mutation of Tyr506 to Phe (corresponding to Tyr381 in the M1 mAChR) has suggested that the tyrosine hydroxyl group is important in agonist (but not antagonist) binding and, in addition, in determining the signaling efficacy of the agonist-stimulated phosphoinositide response (Wess et al., 1991, 1992). In contrast, in the M2 mAChR, the homologous Tyr-Phe mutation affects agonist binding but not the efficacy of the agonist-stimulated adenylyl cyclase inhibition response (Vogel et al., 1997). Because this particular tyrosine residue is conserved in all mAChRs, and has been suggested to play a particular role characteristic to the mAChRs, it is surprising that these two studies gave different results about the functional role of the hydroxyl moiety of the tyrosine. Notably, neither of these studies investigated the role of the benzene ring present in the tyrosine side chain. This moiety is conserved in all of the monoamine receptors and has been proposed to play an important role in receptor function. For example, it has been hypothesized that aromatic residues close to Asp105 in TM 3, including Tyr381, form a cage around the positively charged quaternary nitrogen of acetylcholine (ACh) to stabilize the ion-ion interaction with the conserved aspartate (Trumpp-Kallmeyer et al., 1992).
Other studies have shown that Asn507, in the M3mAChR, which corresponds to an asparagine present in TM 6 of all mAChRs (Asn382 in the M1 mAChR) is involved in antagonist binding to a larger extent than agonist binding and receptor activation (Blüml et al., 1994). Blüml et al. also showed that the Asn507Ser mutant produced a receptor with constitutive activity. This was confirmed and extended by a similar study on the M5 mAChR (Spalding et al., 1998). The investigation by Spalding et al. suggested that Asn459 forms hydrogen bond interactions with carbachol, which stabilizes the receptor in its activated state. Again, there is disagreement between the conclusions drawn by these studies investigating the role of the asparagine residue.
The differences between the investigations described above may originate from the ligands used to probe the effects of the mutations on receptor function. If the number of ligands used is too small, then a structure-activity relationship cannot be easily deduced. In addition, if the compounds used have relatively low binding affinities for the wild-type receptor, there is the risk that mutation of the receptor will cause the ligand to change its mode of binding and lead to inaccurate conclusions being drawn about the receptor-ligand interactions that occur. This problem has been reported to occur with ACh analogs, which tend to have relatively low binding affinities (Page et al., 1995).
In this study, a strategy of alanine-scanning mutagenesis has been used to investigate the roles of the residues from Tyr381 to Val387 in the M1 mAChR. In principle this allows the functional side chain to be removed without perturbing the backbone structure, allowing the role of the side chain to be investigated without gross changes in protein structure.
Because the natural ligand of the mAChRs is ACh, the seven alanine mutants were initially analyzed by using radioligand-binding studies and phosphoinositide turnover assays to measure the effects on ACh binding and ACh-induced functional response, respectively. The residue whose mutation produced the greatest affect on both ACh binding and ACh-induced functional response was Tyr381. Subsequently, this residue was also mutated to phenylalanine so that the role of the functional groups making up the tyrosine residue side chain could be investigated in more detail.
The Tyr381 mutant mAChRs were also characterized further by using a number of ACh analogs, a series of quinuclidine- and azanorbornane-based ligands, and a series of atropine analogs. The quinuclidine- and azanorbornane-based ligands and atropine analogs have higher binding affinities than ACh for mAChRs and are therefore less likely to undergo alterations of their mode of binding when either the receptor or a moiety within a ligand series is changed.
Experimental Procedures
The majority of experimental procedures were as described by Lu et al. (1997).
Site-Directed Mutagenesis and Expression of Muscarinic Receptors.
The DNA sequence coding for the rat M1 mAChR was in a pCD vector (Bonner et al., 1987). Mutations were made by using the Chameleon double-stranded, site-directed mutagenesis kit from Stratagene Inc. (La Jolla, CA) and the mutated sequence was verified by dideoxy sequencing.
Expression of the wild-type and mutated M1 mAChRs in COS-7 cells was the same as described by Lu et al. (1997).
Ligand-Binding Assays.
The production of membrane preparations and ligand-binding assays using (−)[3H]N-methylscopolamine ([3H]NMS) were identical with that described byLu et al. (1997). All binding assays were carried out in 20 mM Na-HEPES, 100 mM NaCl, 1 mM MgCl2, pH 7.5. The assays were performed in triplicate and the unlabeled ligands were diluted in 0.3% (v/v) dimethyl sulfoxide indH2O. Ligand-binding assays using (−)-[3H]quinuclidinyl benzilate ([3H]QNB) were carried out as follows. The binding assays, in 1-ml aliquots, contained [3H]QNB (0.002–1 nM in the saturation binding assays and 80 pM in the competition binding assays), ∼15 μg/ml membrane preparation and unlabeled ligands, diluted in 0.3% (v/v) dimethyl sulfoxide in dH2O, as required. Nonspecific binding was determined by 1 μM atropine except for assays with Asn382Ala-dLoop where 0.1 mM benzilylcholine iodide was used. The assays were performed in triplicate in polystyrene tubes and were incubated at 30°C for 180 min. Termination of the [3H]QNB binding assays was identical with the procedure used for the [3H]NMS binding assays.
Phosphoinositide Turnover Assays.
The experimental procedure used was identical with that described by Jones et al. (1995) and Lu et al. (1997).
Immunocytochemistry.
The experimental procedure used was identical with that described by Lu et al. (1997).
Data Analysis.
The procedures used to carry out data analysis were identical with that described by Lu et al. (1997). Briefly, saturation and competition binding curves were fitted to a one-site model of ligand binding and the Hill equation, respectively, and the phosphoinositide dose-response curves were fitted to a four-parameter logistic function. In a few cases, particularly those in which a high-affinity quaternary antagonist, such as NMS or benzilylcholine, was used to inhibit the binding of the tertiary antagonist, [3H]QNB, a minor population of low-affinity sites (maximum 20%) was detected in addition to the major population of high-affinity sites. A similar phenomenon has been reported previously and the minor population of low-affinity sites attributed to occluded receptors (Brown and Goldstein, 1986). Because the affinities of NMS and the other antagonists at the high-affinity sites were indistinguishable from those measured by direct binding or by competition with [3H]NMS, the high-affinity binding constants obtained by fitting a two-site model of binding (Hulme and Birdsall, 1992) are reported and used in subsequent analysis. Where necessary, ligand-binding data were corrected for the Cheng-Prusoff shift. Signaling efficacy values were calculated using a version of the ternary complex model (Lu et al., 1997; Hulme and Lu, 1998). All of the data analysis was performed using the program SigmaPlot 3.03 (SPSS Inc., Chicago, IL).
Signaling Efficacy Calculations.
Agonist-signaling efficacy values were calculated as described by Lu and Hulme (1999). Briefly, the ratio ([RG] + [ARG])/[GT] calculated from the ternary complex model (De Lean et al., 1980) was fitted to the dose-response data, where [RG] is the concentration of the receptor-G protein binary complex, [ARG] is the concentration of the agonist-receptor-G protein ternary complex, and [GT] is the receptor-accessible concentration of G protein. Unless the basal activity is raised, the contribution of [RG] can be ignored. The calculation uses the agonist-binding constant, K Bin, taken to be the reciprocal of the corrected IC50 value; the agonist potency in the functional response, K Act, which is defined at the reciprocal of the EC50;E Max, the maximum receptor-induced signal relative to the maximum response evoked by ACh at the wild-type receptor; and an estimate of the effective ratio of total receptor concentration to receptor-accessible G protein, [RT]/[GT], denoted R T. In performing these calculations, we have used the value of 20 for R T for the wild-type receptor estimated in a previous study in which the effect of an irreversible blocking agent on the ACh dose-response curve was studied (Lu et al., 1997). Values of R T for the different mutants were calculated from the expression of [3H]NMS or [3H]QNB binding sites. In previous studies we have found that the expression of radioligand-binding sites is well correlated to the functional response (Lu et al., 1997; Lu and Hulme, 1999).
The agonist signaling efficacy parameter isK G · [GT] (denoted K G), whereK G is the apparent bimolecular affinity constant of the G protein for the ensemble of agonist-receptor complexes. K G was computed to reproduce the pEC50 of the phosphoinositide dose-response curve.
For values of
R
T >1, the efficacy parameter,
K
G, was calculated as:
Materials.
The compounds 3-(3-methyl-1,2,4-oxadiazol-5-yl) quinuclidine hydrochloride (L-658,903), 3-(2-methylfuran-4-yl) quinuclidine hydrochloride (L-661,326), 3-(4-methylfuran-2-yl) quinuclidine hydrochloride (L-661,319), (S)-3-(4-methyloxazol-2-yl) quinuclidine hydrochloride (L-683,355), (R)-3-(4-methyloxazol-2-yl) quinuclidine hydrochloride (L-683,356), 3-(3-pyridyl)quinuclidine hydrochloride (L-693,046), and (R)-3-(3-methyl-1,2,4-oxadiazol-5-yl)-1-azabicyclo[2.2.1]heptane hydrochloride (L-698,583) were kindly provided by Dr. Alan Fletcher, Merck Sharp & Dohme Research Laboratories (Harlow, U.K.). Phenylacetyltropine, diphenylacetyltropine,N,N-diethyl-N-methyl-aminoethyl acetate iodide (ACh-N(Et)2),N-methylacetyltropine, and 3-acetoxy-N-methylquinuclidine iodide (Ac-N-Me-Quin) were a gift from Dr. R. B. Barlow (Cumbria, U.K.). Benzilylcholine iodide and ACh-reversed ester (methyl-(N,N-dimethyl-3-amino)propionate methiodide) were synthesized in the laboratory by Dr. E. C. Hulme and their identities were checked by NMR spectroscopy. (−)[3H]NMS (85 Ci/mmol), (−)-[3H]QNB (51 Ci/mmol), and [3H]-myo-d-inositol (80 Ci/mmol) were obtained from Amersham Life Sciences (Little Chalfont, U.K.). All other compounds and materials used were of the highest commercial grade available.
Results
Binding of NMS and QNB
The [3H]NMS and [3H]QNB binding data are summarized in Fig.1. [3H]NMS bound to the wild-type receptor with a K D of 10−10 M. Most of the alanine mutations did not alter the binding of [3H]NMS. However, there was no measurable specific binding of [3H]NMS to Tyr381Ala and Asn382Ala, indicating a K Din excess of 10−8 M. Interestingly, when probed with [3H]QNB, Tyr381Ala showed unaltered affinity (2.3 × 10−11 M) but a reduced number of binding sites (around 16%) relative to wild-type. A competition ligand-binding experiment using unlabeled NMS to inhibit the binding of [3H]QNB showed that theK D of NMS binding to Tyr381Ala was 8.7 × 10−8 M. This implies that when low concentrations of [3H]NMS are used there is a 1000-fold reduction in specific binding to Tyr381Ala relative to the wild-type.
Effects on the binding of [3H]NMS and [3H]QNB caused by alanine substitution mutations of M1 mAChRs. The effect on [3H]NMS and [3H]QNB affinity (A) and receptor expression (B) are shown relative to wild-type M1 mAChR. Wild-type affinity for [3H]NMS and [3H]QNB was 125 ± 5 and 16 ± 4 pM, respectively. There was no measurable binding (N.M.B.) of either [3H]NMS or [3H]QNB to Asn382Ala. Therefore, the expression level shown for Asn382Ala (hatched column) is estimated from the immunocytochemical data, which showed that expression was half of Tyr381Ala. There was also no binding of [3H]NMS to Tyr381Ala and Asn382Ala-dLoop, although a competition radioligand-binding assay using [3H]QNB was able to determine the binding affinity of unlabeled NMS (†). Values are mean ± S.E. of three or more independent experiments with the exception of the immunocytochemical data, which is from one experiment measured in triplicate. Expression levels are expressed relative to a wild-type control included in each transfection. Wild-type expression was 1.15 ± 0.29 pmol/mg protein. The effect of the dLoop mutation is to increase receptor expression level without effecting ligand-binding affinity (Lu et al., 1997). The binding affinities of [3H]NMS and [3H]QNB for dLoop were 100 ± 23 and 14 ± 2 pM, respectively, with the expression 170 ± 20% of wild-type.
When probed with [3H]QNB, Asn382Ala still showed no measurable specific binding of the radioligand. Therefore, a second Asn382Ala mutant M1 mAChR was made that had residues 225 to 353 (dLoop) deleted from the third intracellular loop of the receptor. dLoop mutations have been shown previously to increase the expression of binding sites while having little effect on ligand-binding affinities (Lu et al., 1997). It was possible to measure the binding of [3H]QNB to the Asn382Ala-dLoop mutant. There was a 90-fold reduction in the binding affinity of [3H]QNB relative to wild-type receptor. Direct binding of [3H]NMS could not be measured. A competition binding assay showed that NMS binding to Asn382Ala-dLoop gave a corrected pIC50 of 6.56 ± 0.12.
Immunocytochemistry
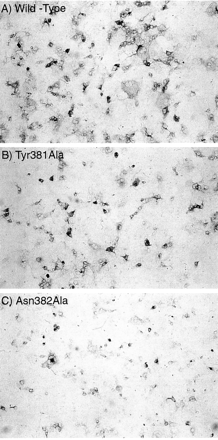
Tyr381Ala and Asn382Ala were the only two alanine-substitution mutations that affected [3H]NMS and [3H]QNB binding. Immunocytochemical staining of transfected COS-7 cells with an anti-C-terminal antibody showed that both mutations caused a reduction in expression of the M1 mAChR protein. Analysis of the reaction product by using a Molecular Dynamics (Sunnyvale, CA) laser-scanning densitometer and Image-Quant software revealed that Tyr381Ala and Asn382Ala expression were 44 ± 7 and 24 ± 5%, respectively, of the wild-type value. The immunocytochemistry data for wild-type, Tyr381Ala, and Asn382Ala are shown in Fig.2.
Immunocytochemistry showing the expression of wild-type, Tyr381Ala, and Asn382Ala M1 mAChRs transiently expressed in COS-7 cells. An antibody directed against a carboxy-terminal epitope was used to probe the transient expression of wild-type (A), Tyr381Ala (B), and Asn382Ala (C) M1 mAChRs in COS-7 cells. Original magnification is 200×. Analysis of the reaction product by using a Molecular Dynamics laser scanning densitometer and Image-Quant software revealed that Tyr381Ala and Asn382Ala expression was 44 ± 7 and 24 ± 5%, respectively, relative to wild-type.
ACh Binding
Competition ligand-binding experiments showed that most of the alanine substitution mutations had little effect on ACh binding (Fig.3A). The mutation that had the largest effect was Tyr381Ala, which reduced ACh binding affinity by 40fold when compared with wild-type. Asn382Ala was studied in context of the dLoop mutation (Asn382Ala-dLoop) gave a 6-fold (p < .001) reduction in ACh binding affinity when compared with wild-type. Leu386Ala had a smaller effect on ACh binding affinity by reducing it 4-fold (p < .01) when compared with wild-type.
The effects on ACh binding, ACh-induced functional response, and ACh efficacy caused by alanine substitution mutations of M1 mAChRs. A, binding affinity of ACh for each of the alanine mutations was measured from three or more experiments and expressed as mean ± S.E. relative to wild-type (corrected pIC50 = 4.89 ± 0.04). The Hill coefficients for wild-type and alanine mutant M1 mAChRs ranged from 0.9 to 1.1. The ACh binding affinity for Asn382Ala could not be calculated because no measurable amounts of radioligand bound to this mutant. B, functional response measured by a phosphoinositide turnover assay was also measured from at least three or more experiments and expressed as mean ± S.E. relative to wild-type (pEC50 = 6.99 ± 0.08) except for Asn382Ala-dLoop (#) that was expressed relative to dLoop (pEC50 = 6.02 ± 0.06). The wild-type basal response was 457 ± 30 dpm and maximal response was 4.4 ± 0.4-fold basal. The alanine mutations had a basal response of 80 ± 3% and a maximal response of 124 ± 7%, when compared with wild-type. C, ACh binding affinity, ACh-induced functional response potency, and receptor expression data were used to calculate ACh efficacy for each mutant. A version of the ternary complex model was used as described by Hulme and Lu (1998). The ACh binding affinity for the Asn382Ala-dLoop mutant was used in the analysis of Asn382Ala. The expression of Asn382Ala was treated as half of Tyr381Ala expression, as suggested by the immunocytochemistry data, i.e., 8% of wild-type (†). The efficacy value calculated for Asn382Ala-dLoop (‡) was compared with dLoop (Lu et al., 1997).
Phosphoinositide Turnover
Four of the mutations caused a less than 4-fold effect on the potency of ACh (Fig. 3B). Tyr381Ala had the largest effect by reducing the potency of ACh by 2750-fold, compared with wild-type. Interestingly, the Asn382Ala mutant was able to produce a phosphoinositide response whose maximum was similar to that of the wild-type receptor and showed a 40-fold reduction in ACh potency. In the context of the dLoop mutation, the difference in potency was 10-fold. Leu386Ala displayed a 30-fold reduction compared with wild-type.
Efficacy Calculations
A receptor-transducer model, such as the ternary complex model, can be used to quantify the effects of mutations on signaling efficacy. An index of agonist-signaling efficacy is provided by the affinity of the G protein for the ensemble of agonist-receptor complexes multiplied by the concentration of receptor-accessible G protein. Subject to the assumption that the catalytic efficiencies of the ternary complexes formed by the different mutants are equivalent to the wild-type, a measure of this can be estimated from a knowledge of: 1) the binding affinity of the agonist, 2) the potency of the agonist in evoking the functional response, and 3) the expression level of the mutant, which determines effective ratio of receptor to G protein (this ratio has been estimated to be 20 for the phosphoinositide response mediated by the wild-type M1 mAChR in COS-7 cells (Lu et al., 1997). As mentioned in Experimental Procedures, the amount of functional receptor was assessed by [3H]NMS and/or [3H]QNB binding, which only detects properly folded and processed receptors (Lu et al., 1997). These calculations allow a correction to be estimated for differences in receptor expression levels (Whaley et al., 1994).
By combining the ACh affinities and potencies, and the receptor expression levels as described previously, the efficacy estimates summarized in Fig. 3C were obtained.
The majority of the alanine mutations affected ACh efficacy by less than 10-fold (increase or decrease) when compared with wild-type. The Tyr381Ala and Leu386Ala mutations reduced ACh efficacy by 50- and 20-fold, respectively, when compared with wild-type. In contrast the estimated efficacy for the Asn382Ala mutant was the same as that of the wild-type receptor, either in the context of the dLoop construct (where its expression was 80%) or the full-length receptor (where its expression was estimated to be 8%). As reported previously, we confirmed that treatment of the cells with propylbenzilylcholine mustard to block 90% of the functional receptors (Lu et al., 1997) caused an approximately 10-fold decrease in the potency of ACh in phosphoinositide turnover experiments, and thus reduced the potency ratio between the Asn382Ala mutant and wild-type receptors to 2-fold (data not shown).
Further Characterization of the Role of Tyr381
Of the seven positions characterized by alanine substitution, Tyr381Ala gave the largest effects on ACh binding and the ACh-induced functional response. To allow the results to be compared with other studies and to differentiate the role of the aromatic ring of the tyrosine residue from that of the hydroxyl group, the Tyr381Phe mutant was made and characterized.
Initially, the binding of [3H]NMS and [3H]QNB was measured and the pK D values of these ligands for the Tyr381Phe mutation were 9.45 ± 0.08 and 11.19 ± 0.02, respectively. The expression of this mutant was measured as 49 ± 2% of wild-type expression (1.15 ± 0.29 pmol/mg protein), from four separate transfections.
As well as the characterization of ACh binding and the ACh-induced functional response, ligand series were used to give more information about the role of Tyr381. These included ACh analogs, azanorbornane- and quinuclidine-based ligands, and atropine analogs. The data from these experiments are summarized in Tables 1, 2, 3, and 4.
The binding of ACh analogs to wild-type and Tyr381 mutant M1mAChRs
The binding of azanorbornane- and quinuclidine-based ligands to wild-type and Tyr381 mutant M1 mAChRs
The binding of atropine analogs to wild-type and Tyr381 mutant M1 mAChRs
Functional response, as measured by the phosphoinositide turnover assays, caused by ligand binding to wild-type and Tyr381 mutant M1 mAChRs
Binding of ACh Analogs
Tyr381Phe and Tyr381Ala both reduced ACh affinity by ∼30-fold when compared with wild-type, where ACh had aK D of 17 μM (Table1). The majority of ACh analogs showed lower affinities than ACh for the wild-type receptor. However, the trends observed with the Tyr381Phe and Tyr381Ala mutations were similar in that both mutations caused carbachol, ACh-reversed ester, and ACh-N(Et)2 affinities to be reduced by ∼6- (p < .001), ∼6- (p < .001), and ∼4-fold (p < .01), respectively. In contrast, tetramethylammonium binding was affected by less than 3-fold when comparing the Tyr381Phe and Tyr381Ala mutant M1mAChRs to wild-type.
Interestingly, the binding affinity of the antagonist benzilylcholine was increased by 2-fold (p < .05) at the Tyr381Phe mutant and decreased by 3-fold (p < .01) at the Tyr381Ala receptor when compared with wild-type.
Binding of Oxotremorine-M and Pilocarpine
The binding affinities of oxotremorine-M and pilocarpine to Tyr381Phe showed a 5- (p < .001) and 3-fold (p < .05) decrease, respectively, when compared with wild-type (Table 1). A 10-fold reduction in their affinities, when compared with wild-type, was observed for both compounds at the Tyr381Ala mutant.
Binding of Azanorbornane- and Quinuclidine-Based Ligands
The azanorbornane- and quinuclidine-based compounds generally had higher binding affinities than ACh for the wild-type M1 mAChR (Table 2). The only exception to this observation was Ac-N-Me-Quin, which had a binding affinity similar to that of ACh.
Head Group Comparisons.
The azanorbornane-based compound (L-698,583) had a 2-fold (p < .01) higher apparent binding affinity for the wild-type M1 mAChR when compared with the quinuclidine-based ligand with the same side chain (L-658,903), although this may reflect the presence of enantiomers of L-658,903. However, the binding affinities of the two compounds were similar for the Tyr381Phe mutant (K D ∼ 1.5 μM), and L-658,903 had an almost 2-fold (p < .05) higher binding affinity than L-698,583 for the Tyr381Ala mutant M1 mAChR.
Side Chain Comparisons.
If the quinuclidine-based ligands that have varying numbers of nitrogens in the side chain are compared, it can be seen that as the number of nitrogens decreases there is little systematic effect on the binding affinity at the wild-type receptor. The binding affinities of the compounds are also similar for the Tyr381Phe mutation (K D ∼ 1.4 μM) except for L-661,319 and L-693,046, which have ∼3-fold higher (p < .001) or ∼2-fold lower (p < .01) binding affinity. By comparing the binding affinities of these compounds for wild-type and Tyr381Phe receptors and so examining the consequences of removal of the Tyr381 hydroxyl group, it can be seen that there may be a trend (p = .03) related to the number of nitrogens in the side chain, although the effect is not large.
The ligands binding to Tyr381Ala show a more pronounced trend according to the number of nitrogens in their side chain. As the number of nitrogens in the side chain decreases, the binding affinity of the compounds increases. If the effects of removing the benzene ring are compared by measuring the change in binding affinity between Tyr381Phe and Tyr381Ala, it can be seen that the number of nitrogens in the side chain is a major determinant of the change measured (p< .001).
The quinuclidine-based ligands with one nitrogen in the side chain (L-683,355, L-683,356, and L-693,046) all showed results similar to those obtained for ACh; the removal of the Tyr381 hydroxyl group (wild-type to Tyr381Phe) reduced the ligand-binding affinity by 2- (p < .01), 3- (p < .001), and 4-fold (p < .01), respectively, whereas removal of the benzene ring (Tyr381Phe to Tyr381Ala) did not significantly alter the ligand-binding affinity. This pattern was also seen with Ac-N-Me-Quin.
Binding of Atropine Analogs
The use of a series of atropine-based ligands allowed a number of comparisons to be made, and the data obtained from analyzing the binding affinities of these compounds for wild-type and Tyr381 mutant M1 mAChRs (Table 3) enabled a structure-activity relationship to be built up. NMS, of all the atropine-based compounds looked at, has the highest binding affinity for the wild-type receptor (K D = 130 pM), and it can be seen that altering moieties in both the tropane ring and the tropic acid side chain reduced this ligand-binding affinity. The Tyr381 mutations also affected the binding affinities of these compounds, although a general observation can be made: the change in binding affinity observed between the wild-type and Tyr381Phe mutant, for the atropine-based ligands, tends to be smaller than the change measured between Tyr381Phe and Tyr381Ala.
Head Group Comparisons.
The atropine-based ligands allowed investigation of differences between the binding of antagonists containing a quaternary or a tertiary nitrogen. If the binding affinity of NMS is compared with that obtained for (−)scopolamine (both compounds containing an epoxide-oxygen), it can be seen that removal of a methyl group attached to the nitrogen (quaternary to tertiary) results in a 2-fold decrease (p < .05) in binding affinity for the wild-type receptor. Similar results were obtained for the comparisons between N-methylatropine and atropine (p < .05), and N-methylhomatropine and homatropine (p < .01), with both pairs of compounds displaying a 2-fold decrease in binding affinity for the wild-type M1 mAChR. At the Tyr381Phe mutation, the 2-fold higher affinities of the quaternary analogs were preserved in the case of atropine (p < .05) and (−)scopolamine (p < .001), but not homatropine. These differences were abolished by the Tyr381Ala mutation.
Side Chain Comparisons (Hydroxyl Group).
The hydroxylmethyl group found on the tropic acid side chain of NMS and other atropine-based ligands is also important for binding. If the binding ofN-methylatropine is compared withN-methylhomatropine and atropine to homatropine it can be seen that the binding affinity for the wild-type receptor is reduced 70- to 90-fold when the methylene group is removed, shortening the side chain bearing the hydroxyl group. At the Tyr381Phe mutant similar effects were observed. At the Tyr381Ala receptor significant differences are still seen, although their magnitude is reduced.
The comparison of either atropine and phenylacetyltropine or homatropine and phenylacetyltropine gives some information about what happens when the hydroxyl group is completely removed. The latter pair will be considered because in the first pair the methylene group is removed as well. At the wild-type receptor, removing the hydroxyl group causes a 5-fold reduction (p < .001) in the binding affinity (homatropine to phenylacetyltropine). This difference is almost identical at the Tyr381Phe mutant (4-fold; p < .001) but is not significant when binding to the Tyr381Ala M1 mAChR.
Side Chain Comparisons (Benzene Ring).
The presence of the benzene ring at the end of the tropic acid side chain is necessary for high-affinity binding by atropine-based compounds. The effect of the removal of the ring, approximated by comparing the binding of phenylacetyltropine and N-methylacetyltropine, reveals a 20-fold decrease in the ligand-binding affinity at the wild-type receptor. The change was 50-fold at both the Tyr381Phe and Tyr381Ala mutant M1 mAChRs. In contrast, the addition of a benzene ring to the side chain increases ligand-binding affinities. If diphenylacetyltropine is compared with phenylacetyltropine and benzilyltropine to homatropine it can be seen that the addition of an extra benzene ring increased the ligand-binding affinities to the wild-type receptor by 30- and 240-fold, respectively. At the Tyr381Phe mutant, the increases observed were 100- and 1510-fold, whereas at the Tyr381Ala receptor there were 10- and 1230-fold increases.
The absolute binding affinity of diphenylacetyltropine increased by 2-fold (p < .01) and decreased by 10-fold (p < .01) at the Tyr381Phe and Tyr381Ala M1 mAChRs when compared with the wild-type value (K D of 8 nM). In contrast, benzilyltropine binding is not significantly affected by the Tyr381 mutations. The results obtained for benztropine, which has the two benzene rings attached to the tropane ring via an ether linkage, show that its binding affinities for wild-type and Tyr381Phe receptors are similar (K D values ∼ 400 pM), whereas at the Tyr381Ala M1 mAChR, its binding affinity is reduced 7-fold (p < .001) when compared with wild-type. This observation of increased or unchanged affinities for binding to the Tyr381Phe mutant differs from the general trend, i.e., the Tyr381Phe mutation causes a decrease in the binding affinity of most ligands when compared with their binding affinity for the wild-type receptor.
Functional Response Induced by ACh Analogs
To try to get information about the role of Tyr381 in receptor activation, a selection of the agonists used in the radioligand-binding studies was used in phosphoinositide turnover assays (Table4). Tyr381Phe and Tyr381Ala caused a 70- and 2750-fold reduction in ACh potency in the functional response, respectively, when compared with the wild-type response (EC50 = 100 nM), without reducing the maximum response. The basal measurements for each mutant were slightly reduced.
The ability of two other ACh analogs (ACh-reversed ester and tetramethylammonium) to produce a functional response at the wild-type and Tyr381 mutant receptor was measured. The data showed that altering the ACh structure had significant effects on the functional response produced by the wild-type receptor. ACh-reversed ester and tetramethylammonium showed a 50- and 1320-fold reduction in potency, and both compounds only gave a maximum response of ∼85% when compared with the data for ACh.
The effect of the Tyr381Phe mutation on the functional response caused by binding of ACh-reversed ester and tetramethylammonium was to produce a 20- and 7-fold (p < .001) reduction in potency when compared with wild-type. Although the maximum response produced by ACh-reversed ester binding to Tyr381Phe was similar to its wild-type response, tetramethylammonium showed an almost 2-fold decrease (p < .01) in the maximum response elicited from the Tyr381Phe mutant compared with the wild-type receptor. A 2-fold decrease (p < .05) in maximum response and 200-fold decrease in potency was observed for ACh-reversed ester activation of Tyr381Ala when compared with the wild-type response. However, no measurable functional response was evoked by tetramethylammonium binding to the Tyr381Ala M1 mAChR.
Functional Response Induced by Azanorbornane- and Quinuclidine-Based Ligands
The azanorbornane-based ligand, L-698,583, and quinuclidine-based ligand, L-658,903, were also used in functional studies (Table 4). L-698,583 and L-658,903 both showed increased potencies [130- and 6-fold (p < .001), respectively] and similar maximal responses when compared with ACh. The Tyr381Phe mutation reduced the potency of the functional response produced by L-698,583 and L-658,903 by 90- and 30-fold, respectively, although both compounds still gave a similar maximal response to that evoked by ACh. The Tyr381Ala mutation had an effect on the maximal response produced by L-658,903 (reducing it to 50% of the wild-type response), whereas L-698,583 was able to give a larger maximal response equivalent to 118% of the wild-type ACh response. However, the potencies of L-698,583 and L-658,903 were reduced by the Tyr381Ala mutation 10,000- and 2,700-fold, respectively, when compared with their potencies at the wild-type receptor.
The potency of L-658,903 in the functional response evoked by the Tyr381Ala mutant appears to be 5-fold lower than the binding affinity. However, L-658,903 is a weak partial agonist at Tyr381Ala, therefore the results from the phosphoinositide turnover experiments have relatively large errors.
Efficacy Calculations
The data obtained from the phosphoinositide turnover experiments using wild-type and Tyr381 mutant receptors were combined with the binding affinity data to calculate efficacy values (Table5). It can be seen that both the ACh analogs and the quinuclidine L-658,930 have lower efficacy than ACh at the wild-type receptor. However, the azanorbornane L-698,583 has an efficacy at the wild-type receptor that is comparable to that of ACh. The other observation that can be made is that the Tyr381Ala mutation affects compound efficacy to a larger extent (>10-fold) than Tyr381Phe when compared with the wild-type receptor.
Effects on compound efficacy caused by Tyr381 mutant M1 mAChRs
Discussion
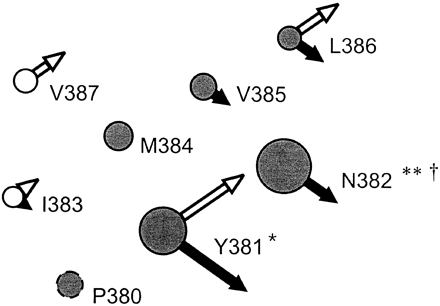
In this study alanine-scanning mutagenesis was used to investigate the residues from Tyr381 to Val387, in TM 6, of the rat M1 mAChR. The outcome is summarized in Fig.4. The residues whose mutation affected ligand binding form a subset of the positions accessible from within the binding cleft in the homologous sequence in the D2 dopamine receptor (Javitch et al., 1998). This would be consistent with (does not prove) the existence of direct interactions between some of these residues and the ligands.
Scanning mutagenesis of residues in TM 6 of the M1 mAChR. The figure shows the amino acids from Pro380 to Val387. In this study Tyr381 to Val387 were sequentially mutated to alanine. Pro380 was not mutated in this study but is conserved in all rhodopsin-like receptors (circle shown with dashed outline). The diameter of each circle represents the effect of the mutation on the expression level relative to the wild-type; Log (expressionwild-type/expressionmutant) + 1. Filled arrows represent the effect on ACh-receptor binary complex formation; wild-type corrected pIC50 − mutant corrected pIC50. Open arrows represent the effect on ACh efficacy; Log (efficacywild-type) − Log (efficacymutant). Right pointing arrows represent a decrease relative to wild-type, and left pointing arrows an increase relative to wild-type. Change in NMS affinity relative to wild-type is shown by: ∗, 100- to 1000-fold reduction; ∗∗, >1000-fold reduction. Change in QNB affinity relative to wild-type is shown by: †, 10- to 100-fold reduction. The ligand-binding data for Asn382 was determined using the Asn382Ala-dLoop mutant. The filled circles represent residues in the homologous position to those shown by Javitch et al. (1998), to be accessible by sulfhydryl-specific reagents.
The two-state model of receptor activation implies that mutations can change either the affinity constant, which determines the formation of the ground state binary complex, or the isomerization constant, which governs the resultant conformational change. In the case of the M1 mAChR, previous studies have suggested that any reduction in ACh affinity arising from inhibition of the conformational change is unlikely to be greater than approximately 2-fold (Hulme and Lu, 1998; Lu and Hulme, 1999). Thus larger effects are likely to reflect changes in the ground state binding constant. In contrast, changes in the signaling efficacy reflect an alteration in the stability of the active agonist-receptor-G protein complex with respect to the ground state agonist-receptor binary complex.
Only Tyr381 and Asn382 were involved in the binding of [3H]NMS and [3H]QNB. Tyr381 discriminates between these radiolabeled antagonists, indicating that they bind in different orientations and/or interact with different residues. However, both compounds may form important interactions with Asn382, in agreement with studies on the homologous mutation in the M3 mAChR (Blüml et al., 1994).
The reduction in receptor expression levels caused by Tyr381Ala and Asn382Ala suggest that they may also form intramolecular interactions that stabilize receptor folding (Lu et al., 1997). When these interactions are disrupted, incorrect folding of the receptor protein increases, probably resulting in increased degradation. A similar observation was made for Asn507Ala (Blüml et al., 1994) in the M3 mAChR.
Most of the alanine substitution mutations had little effect on ACh binding or the ACh-induced functional response. The mutations that did affect the receptor’s response to ACh were Tyr381Ala, Asn382Ala (Asn382Ala-dLoop), and Leu386Ala. However, Asn382Ala did not affect ACh efficacy, implying that Asn382 does not mediate the process of receptor activation by ACh. These results differ from Spalding et al. (1998), who suggest that the homologous asparagine in the M5 mAChR plays a role in receptor activation by carbachol. The role of Asn382 in receptor activation seems to be determined by ligand structure (S.D.C.W., unpublished observations). In contrast, a very large effect on receptor function resulted from the Tyr381Ala mutation, indicating that Tyr381 plays a major role in M1 mAChR activation.
Additional characterization of the Tyr381Ala mutant, combined with studies of Tyr381Phe, gave additional insight into the function of Tyr381. The binding data suggests that the Tyr381 hydroxyl group does not play a large role in the binding of NMS or QNB. In contrast, the benzene ring of Tyr381 seems to make a significant interaction with NMS.
The atropine analog data also suggests that the benzene ring of Tyr381 forms strong interactions with the most potent of these ligands in the ground state of the receptor. It is possible that the benzene ring may interact with the tropane ring. Comparing compounds with different substitutions on the tropane ring, e.g., a quaternary/tertiary nitrogen, there are small differences in binding affinities at the wild-type receptor that are still present at the Tyr381Phe mutant. These differences are abolished by the Tyr381Ala mutation. Therefore, the benzene ring may be interacting close to the nitrogen in the tropane ring.
The side chain hydroxyl group and the terminal benzene ring probably do not make direct interactions with Tyr381 because differences in affinities between analogs with varying substitutions in these positions remain when Tyr381 is mutated.
The affinity of N-methylacetyltropine, which simply has an acetoxy group attached to the tropane ring, is reduced by the removal of the Tyr381 hydroxyl group to a similar extent to atropine. The hydroxyl group of Tyr381 may interact weakly with the carbonyl- or ether-oxygen of atropine and closely related analogs.
The azanorbornane- and quinuclidine based ligands with either an acetyl group (Ac-N-Me-Quin) or one nitrogen atom (L-683,355; L-683,356; L-693,046) in the side chain have a similar binding pattern to N-methylacetyltropine in that removal of the Tyr381 hydroxyl group reduces ligand affinity whereas subsequent removal of the benzene ring does not.
The addition of a second nitrogen atom or removal of all the nitrogen atoms in the side chain may cause the mode of binding used by these ligands to change. The addition of a second strong hydrogen bond acceptor to the side chain of these ligands induces a positive interaction with the Tyr381 benzene ring. In contrast, removal of the strong hydrogen bond acceptors abolishes the interaction made by the Tyr381 hydroxyl group and causes the benzene ring interaction to become restrictive. Deletion of the Tyr381 benzene ring actually increases the affinity of these ligands, perhaps by allowing them greater access to a nonpolar binding domain.
The presence of an extra methylene moiety in the quinuclidine, when compared with the azanorbornane head group causes a 10-fold decrease in wild-type signaling efficacy. This difference persists at both the Tyr381 mutant receptors. Thus the difference is not due to interactions with Tyr381. However, the Tyr381 benzene ring remains important for forming an interaction with these ligands in the activated state of the receptor, allowing the possibility that it interacts with another region of the head group. The two high-affinity azanorbornane- and quinuclidine-oxadiazole ligands seem to bind to both the hydroxyl group and the benzene ring of Tyr381 in the ground state of the receptor. Both interactions are strengthened in the activated state of the receptor.
The results obtained for ACh, ACh-reversed ester, and carbachol show that removal of the Tyr381 hydroxyl group substantially reduced ligand-binding affinity. Subsequent removal of the Tyr381 benzene ring did not affect ligand affinity: all three compounds had similar binding affinities for both of the Tyr381 mutant receptors. In contrast, removal of the benzene ring but not the hydroxyl group strongly reduced the signaling efficacy of ACh and ACh-reversed ester.
The simplest hypothesis consistent with the binding and functional data for these compounds is as follows. In the ground state of the receptor, the hydroxyl group of Tyr381 forms a hydrogen bond interaction with the ester moiety present in the side chain of ACh. This bond is preserved in the activated state of the receptor, but is not the primary interaction driving activation. Rather, it seems that it is the benzene ring of Tyr381 that is primarily involved in forming and/or stabilizing the activated state. However, the ACh analogs discussed so far do not suggest with which ligand moiety the benzene ring interacts.
Tetramethylammonium gives some insight into the interaction that the Tyr381 benzene ring may form. The Tyr381 mutations reduce the affinity of tetramethylammonium by less than 3-fold, in agreement with the hypothesis described above, because it lacks a side chain able to form hydrogen bonds. The removal of the hydroxyl group does not affect the efficacy of tetramethylammonium, again in agreement with the hypothesis. However, removal of the benzene ring abolishes the functional response, implying a large decrease in efficacy. This data is consistent with the proposal that the benzene ring of Tyr381 forms an interaction with tetramethylammonium, and by implication with the positively charged head group of ACh, in the activate state of the receptor. Tyrosine residues are known to be able to form cation-π interactions (Scrutton and Raine, 1996; Ma and Dougherty, 1997), suggesting a possible chemical basis for this interaction. A cation-π interaction may also take place between the Tyr381 benzene ring and the head groups of the azanorbornane- and quinuclidine-based ligands and the atropine analogs. A recent study has also implicated a cation-π interaction in the activation of the nicotinic ACh receptor (Zhong et al., 1998).
Mutation of Tyr381 preserves the binding of QNB and other benzilates, although it has large effects on the binding of atropine analogs. Asn382Ala has undiminished signaling efficacy. These findings argue against the induction of major structural changes by the mutations, at least in the receptors that undergo correct cellular processing. Thus we propose that both Tyr381 and Asn382 are primary ligand contact residues. Leu386 may play a supporting ‘second-shell’ role. Within this domain of TM 6, Tyr381 is the most important residue for activation of the receptor by ACh.
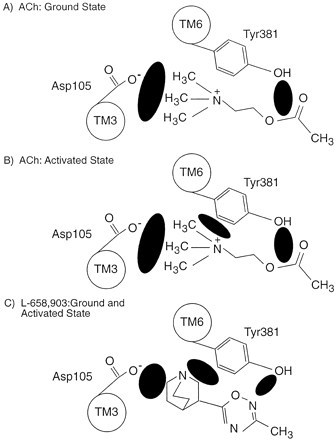
Figure 5 illustrates a working hypothesis for Tyr381 function in the M1 mAChR. When binding ACh, the M1 mAChR’s natural ligand, the hydroxyl group probably forms a hydrogen bond with the ester linkage oxygens, in the ground state of the receptor. This conclusion agrees with previous investigations in which the homologous tyrosines in the M2 and M3 mAChRs were mutated to phenylalanine (Wess et al., 1992; Vogel et al., 1997).
Summary of the interactions that Tyr381 may be making in the ground and activated state of the receptor. Cartoons showing the interactions Tyr381 makes with ACh and bulkier and/or more complex ligands, e.g., L-658,903, in the ground and activated state of the receptor. Apart from Tyr381, the conserved aspartate (Asp105) in TM 3, which has been shown to play an important role in ligand binding, is also displayed (Page et al., 1995). A, in the ground state of the receptor, ACh is proposed to form a hydrogen bond interaction with the hydroxyl group of Tyr381 via the ester moiety present in its side chain, and there is an interaction formed between Asp105 and the positively charged head group. B, in the activated state of the receptor, these interactions are preserved and there are additional interactions proposed between the benzene ring of Tyr381 and the head group of ACh. C, in the case of L-658,903, both the Tyr381 hydroxyl group and benzene ring as well as Asp105 make interactions with the ligand in both the ground and activated states of the receptor. The interactions described are shown in the figure by filled ellipses.
In contrast, the benzene ring of Tyr381 may make contact with the head group quaternary nitrogen of ACh, possibly by a cation-π interaction, to stabilize the activated state of the receptor. This finding agrees with the modeling that suggests that the phenyl moiety found in this position in cationic amine receptors is involved in caging the positively charged head group while it interacts with the TM 3 aspartate (Trumpp-Kallmeyer et al., 1992). A process of ligand capture by charge and polar interactions followed by a conformational change driven by aromatic cage formation may underlie activation of the mAChRs by ACh.
Acknowledgments
We thank Dr. Noel Buckley (University of Leeds, Leeds, U.K.) for the rat M1 mAChR in a pCD expression vector, Dr. Alan Fletcher (Merck Sharp & Dohme Research Laboratories, UK) for providing the L-compounds, Dr. Stephen Freedman (Athena Neurosciences Inc.) for his support in developing the research strategy used, and Dr. Nigel Birdsall for his comments on this manuscript.
Footnotes
- Received March 11, 1999.
- Accepted July 30, 1999.
-
Send reprint requests to: Dr. E.C. Hulme, Division of Physical Biochemistry, National Institute for Medical Research, The Ridgeway, Mill Hill, London, NW7 1AA UK. E-mail: ehulme{at}nimr.mrc.ac.uk
-
1 This work was supported by the Medical Research Council (UK) and a Merck Sharp & Dohme collaborative studentship (S.D.C.W.).
Abbreviations
- TM
- transmembrane domain
- mAChR
- muscarinic acetylcholine receptor
- ACh
- acetylcholine
- ACh-reversed ester
- methyl-(N, N-dimethyl-3-amino)propionate methiodide
- ACh-N(Et)2
- N,N-diethyl-N-methyl-aminoethyl acetate iodide
- Ac-N-Me-Quin
- 3-acetoxy-N-methylquinuclidine iodide
- L-658,903
- 3-(3-methyl-1,2,4-oxadiazol-5-yl)quinuclidine hydrochloride
- L-661,326
- 3-(2-methylfuran-4-yl)quinuclidine hydrochloride
- L-661,319
- 3-(4-methylfuran-2-yl)quinuclidine hydrochloride
- L-683,355
- (S)-3-(4-methyloxazol-2-yl)quinuclidine hydrochloride
- L-683,356
- (R)-3-(4-methyloxazol-2-yl)quinuclidine hydrochloride
- L-693,046
- 3-(3-pyridyl)quinuclidine hydrochloride
- L-698,583
- (R)-3-(3-methyl-1,2,4-oxadiazol-5-yl)-1-azabicyclo[2.2.1]heptane hydrochloride
- NMS
- N-methylscopolamine
- QNB
- quinuclidinyl benzilate
- The American Society for Pharmacology and Experimental Therapeutics