Abstract
The metabotropic glutamate receptor subtype 5 (mGlu5) activates calcium mobilization via binding of glutamate, the major excitatory neurotransmitter in the central nervous system. Allosteric modulation of the receptor has recently emerged as a promising alternative method of regulation to traditional regulation through orthosteric ligands. We now report three novel compounds that bind to the allosteric 2-methyl-6-(phenylethynyl)-pyridine (MPEP) site on mGlu5 but have only partial inhibition or no functional effects on the mGlu5 response. Two of these compounds, 2-(2-(3-methoxyphenyl)ethynyl)-5-methylpyridine (M-5MPEP) and 2-(2-(5-bromopyridin-3-yl)ethynyl)-5-methylpyridine (Br-5MPEPy), act as partial antagonists of mGlu5 in that they only partially inhibit the response of this receptor to glutamate. The third compound, 5-methyl-6-(phenylethynyl)-pyridine (5MPEP), acts as a neutral allosteric site ligand that binds to the MPEP site and has no effects alone. However, 5MPEP blocks the effects of both the allosteric antagonist MPEP and potentiators 3,3′-difluorobenzaldazine and 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB). This compound also blocks depolarization effects of both MPEP and CDPPB in neurons in the subthalamic nucleus. These novel compounds provide valuable new insight into the pharmacology of allosteric sites on G protein-coupled receptors and provide valuable new tools for determining the effects of allosteric site ligands in native systems.
Glutamate, the major excitatory neurotransmitter in the central nervous system, exerts its effects through both ionotropic and metabotropic glutamate (mGlu) receptors. The mGlu receptors are members of the family C G protein-coupled receptors (GPCRs), distinguished from other families of GPCRs by a large extracellular N-terminal agonist binding site (for reviews, see Conn and Pin, 1997; Pin and Acher, 2002). The mGlu receptors provide a mechanism by which glutamate can modulate or fine tune activity at the same synapses at which it elicits fast synaptic responses. There are eight known members of the mGlu family, divided into three groups based on sequence homology, pharmacology, and coupling to intracellular signaling pathways. Group I mGlu receptors (mGlu1 and mGlu5) are primarily localized at postsynaptic sites and couple to Gαq and increases in intra-cellular calcium. Group II (mGlu2 and mGlu3) and group III (mGlu4, mGlu6, mGlu7, and mGlu8) mGlu receptors are predominantly presynaptic and couple to Gαi/o and associated effectors, including inhibition of adenylyl cyclase and various ion channels.
The group I receptor mGlu5 has been implicated in a number of normal physiological processes in the central nervous system. Previous studies suggest that selective agonists and antagonists of mGlu5 could have utility for treatment of a number of central nervous system disorders, including pain (Varney and Gereau, 2002), anxiety disorders (Spooren and Gasparini, 2004; Swanson et al., 2005), Parkinson's disease (Marino and Conn, 2002a), addiction (Kenny and Markou, 2004), and schizophrenia (Marino and Conn, 2002b; Moghaddam, 2004). Unfortunately, it has been difficult to develop compounds that act as selective ligands at the orthosteric glutamate binding site of mGlu5 or other individual mGlu subtypes that have properties that are likely to be suitable for development of therapeutic agents. However, a major breakthrough in discovery of selective antagonists for mGlu5 came with the discovery of highly selective allosteric mGlu5 receptor antagonists, including 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and related compounds (for reviews, see Spooren et al., 2001; Gasparini et al., 2002). These compounds do not interact with the orthosteric glutamate binding site but bind to an allosteric site in the seven transmembrane-spanning domain of mGlu5 to inhibit coupling of the receptor to GTP-binding proteins (G proteins). Discovery of selective mGlu5 receptor antagonists has had a major impact on our understanding of the physiological roles of this receptor and has allowed studies that suggest that antagonists of this receptor have potential as novel therapeutic agents for treatment of pain and anxiety disorders. More recently, we have reported discovery of three distinct series of compounds that act as highly selective allosteric potentiators of mGlu5. These are exemplified by 3,3′-difluorobenzaldazine (DFB) (O'Brien et al., 2003), N-[4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl]-2-hydroxybenzamide (O'Brien et al., 2004), and 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) (Lindsley et al., 2004; Kinney et al., 2005). These compounds have no agonist activity by themselves, but they act at an allosteric site to potentiate glutamate-induced activation of mGlu5 in transfected cell lines. Recent studies with CDPPB suggest that this allosteric potentiator of mGlu5 has effects in animal models that are consistent with potential antipsychotic activity of these compounds (Kinney et al., 2005).
Discovery of allosteric modulators of mGlu5 represents an exciting advance in demonstrating the potential of this approach for developing novel research tools and therapeutic agents that regulate activity of specific mGluR subtypes. However, little is known about the pharmacological properties of specific domains involved in the action of different classes of mGlu receptor allosteric regulators or the range of activities that compounds in a given structural class can have on these sites. We have developed a large range of structural analogs of the mGlu5 receptor allosteric site antagonist MPEP (Alagille et al., 2005a,b). Most of these MPEP analogs act as potent allosteric antagonists of mGlu5. However, we now report three novel compounds in this series that bind to the MPEP site on mGlu5 but that have only partial inhibition or no functional effects on the mGlu5 response. Two of these compounds, 2-(2-(3-methoxyphenyl)ethynyl)-5-methylpyridine (M-5MPEP) and 2-(2-(5-bromopyridin-3-yl)-ethynyl)-5-methylpyridine (Br-5MPEPy) act as “partial antagonists” of mGlu5 in that they only partially inhibit the response of this receptor to glutamate. These are clearly distinct from partial agonists at orthosteric sites in that they do not activate the receptor. The third compound, 5-methyl-6-(phenylethynyl)-pyridine (5MPEP), acts as a neutral allosteric site ligand that binds to the MPEP site and has no effects alone. However, 5MPEP blocks the effects of both the allosteric antagonist MPEP and potentiators DFB and CDPPB. Furthermore, this compound blocks electrophysio-logical effects of both MPEP and CDPPB in neurons in the subthalamic nucleus. These novel compounds provide valuable new insight into the pharmacology of allosteric sites on GPCRs. Furthermore, 5MPEP and related compounds provide valuable new tools for determining the effects of allosteric site ligands in native systems.
Materials and Methods
Materials. MPEP and DFB were obtained from commercial sources (Tocris Cookson Inc., Ellisville, MO). CDPPB and 5MPEP were synthesized as described previously (Lindsley et al., 2004; Alagille et al., 2005a,b). M-5MPEP and Br-5MPEPy and their precursors were synthesized as follows.
5-Methyl-2-trimethylsilanylethynyl-pyridine. To a solution of 2-bromo-5-methylpyridine (17.4 mmol) in 50 ml of degassed triethylamine was added trimethylsilylacetylene (19.1 mmol), CuI (1.74 mmol), and trans-dichlorobis(triphenylphosphine)palladium (1.74 mmol). The resulting solution was stirred at room temperature overnight under N2 atmosphere. The black solution was then hydrolyzed with 30 ml of H2O and extracted with diethyl ether (3 × 30 ml). Purification of the residue by column chromatography (hexane/diethyl ether 9/1) provided the desired compound in 81% yield as brown oil. 1H NMR (CDCl3) δ ppm: 0.08 (s, 9H, CH3); 2.30 (s, 3H, CH3); 7.41 (d, 1H, J = 7.5 Hz, CHAr), 7.59 (d, 1H, J = 7.5 Hz, CHAr); 8.41 (s, 1H, CHAr). 13C NMR (CDCl3) δ ppm: 0.0 (3C, CH3); 18.9 (1C, CH3); 94.0 (1C, C≡C); 104.7 (1C, C≡C); 126.1 (1C, CHAr); 132 (1C, Cq); 135.2 (1C, CHAr); 141.6 (1C, Cq); 150.7 (1C, CHAr).
2-(3-Methoxyphenylethynyl)-5-methylpyridine (M-5MPEP). To a solution of 5-methyl-2-trimethylsilanylethynylpyridine (5.32 mmol) in 25 ml of degassed N,N-dimethylformamide was added successively 3-bromoanisole (6.96 mmol), CuI (0.57 mmol), triethylamine (21.2 mmol), and trans-dichlorobis(triphenylphosphine)palladium (29 mmol). The resulting mixture was warmed to 70°C, tetra-n-butyl ammonium fluoride (5.85 mmol) was added drop-wise, and the reaction was stirred at this temperature for 2 h. After cooling, 20 ml of H2O was added, and the resulting solution was extracted with ethyl acetate (4 × 20 ml). The organic layer was washed with saturated NaCl (3 × 20 ml), dried over Na2SO4, and evaporated to dryness. Purification of the residue by column chromatography provided M-5MPEP in 52% yield as a yellow oil. HCl salts were prepared by adding 2 M HCl/diethyl ether to a solution of free base in ethyl acetate and isolated by suction filtration. 1H NMR (CDCl3) δ ppm: 2.20 (s, 3H, CH3); 3.67 (s, 3H, CH3); 6.79 (ddd, 1H, J = 8.5, 2.5, 1.0 Hz, CHAr); 7.01-7.02 (m, 1H, CHAr); 7.07 (dt, 1H, J = 8.5, 1.0 Hz, CHAr); 7.13 (t, 1H, J = 8.5 Hz, CHAr); 7.29 (d, 1H, J = 7.5 Hz, CHAr); 7.33 (dd, 1H, J = 7.5, 2.5 Hz, CHAr); 8.32 (s, 1H, CHAr). 13C NMR (CDCl3) δ ppm: 18.8 (1C, CH3); 55.6 (1C, CH3); 88.8 (1C, C≡C); 88.9 (1C, C≡C); 115.9 (1C, CHAr); 116.9 (1C, CHAr); 123.8 (1C, Cq); 124.6 (1C, CHAr); 127.0 (1C, CHAr); 129.6 (1C, CHAr); 133.1 (1C, Cq); 137.0 (1C, CHAr); 140.8 (1C, Cq); 150.9 (1C, CHAr); 159.6 (1C, Cq). HCl salt melting point 171-172°C. Anal. (C15H13NO·HCl·0.2 H2O) C, H, N.
3-(6-Methylpyridin-2-ylethynyl)-5-bromopyridine (Br-5M-PEPy). To a solution of 5-methyl-2-trimethylsilanylethynylpyridine (5.32 mmol) in 25 ml of degassed N,N-dimethylformamide was added successively 3,5-dibromopyridine (6.96 mmol), CuI (0.57 mmol), triethylamine (21.2 mmol), and trans-dichlorobis(triphenylphosphine)palladium (29 mmol). The resulting mixture was warmed to 70°C, tetra-n-butyl ammonium fluoride (5.85 mmol) was added drop-wise, and the reaction was stirred at this temperature for 2 h. After cooling, 20 ml of H2O was added, and the resulting solution was extracted with ethyl acetate (4 × 20 ml). The organic layer was washed with saturated NaCl (3 × 20 ml), dried over Na2SO4, and evaporated to dryness. Purification of the residue by column chromatography provided Br-5MPEPy in 41% yield as a clear oil. HCl salt was prepared by adding 2 M HCl/diethyl ether to a solution of free base in ethyl acetate and isolated by suction filtration. 1H NMR (CDCl3) δ ppm: 2.24 (s, 3H, CH3); 7.32 (d, 1H, J = 7.5 Hz, CHAr); 7.38 (dd, 1H, J = 7.5, 2.5 Hz, CHAr); 7.86 (t, 1H, J = 2.5 Hz, CHAr); 8.34 (s, 1H, CHAr); 8.50 (d, 1H, J = 2.5 Hz, CHAr); 8.59 (d, 1H, J = 2.5 Hz, CHAr). 13C NMR (CDCl3) δ ppm: 18.9 (1C, CH3); 83.6 (1C, C≡C); 93.3 (1C, C≡C); 120.3 (1C, Cq); 121.4 (1C, Cq); 127.2 (1C, CHAr); 133.9 (1C, Cq); 137.0 (1C, CHAr); 139.6 (1C, Cq); 141.2 (1C, CHAr); 150.4 (1C, CHAr); 150.7 (1C, CHAr); 151.1 (1C, CHAr). HCl salt melting point 166-168°C. Anal. (C13H9N2·HCl·0.6 H2O) C, H, N.
Rat Cortical Astrocytes. Rat cortical astrocytes were prepared as described by Peavy et al. (2001). In brief, neocortices from 2- to 4-day-old Sprague-Dawley rat pups were dissected and dissociated in DMEM by trituration with 1-ml pipette tips. The cells were then centrifuged and resuspended in DMEM (containing 1 mM sodium pyruvate, 2 mM l-glutamine, and 100 units/ml penicillin/streptomycin) supplemented with 10% FBS in T75 tissue culture flasks; the medium was changed the next day. Cell cultures were maintained at 37°C in an atmosphere of 95% air, 5% CO2 for 6 to 8 days. Cells were shaken overnight (280-310 rpm) to remove oligodendrocytes and microglia.
Calcium Fluorescence Assay. Secondary astrocytes were trypsinized and replated into poly-d-lysine-coated 384 well plates (Greiner Bio-One, Longwood, FL) at 10,000 cells/well in 20 μl of growth medium (DMEM containing 10% FBS, 20 mM HEPES, 2 mM l-glutamine, and antibiotic/antimycotic). On the 2nd day, the medium was changed to growth medium and G-5 supplement (Invitrogen, Carlsbad, CA) containing epidermal growth factor (10 ng/ml), basic fibroblast growth factor (5 ng/ml), insulin (5 μg/ml), and other factors. The cells were nearly confluent within 2 days and resembled the polygonal astrocytic appearance in vivo. The 4th day, approximately 20 h before experiments, the medium was changed to glutamine-free DMEM containing 5% dialyzed FBS, 20 mM HEPES, and antibiotic/antimycotic. On day 5, medium was removed from the plate, and the cells incubated with 20 μl of 1 μM Fluo-4 acetoxymethyl ester (Invitrogen) in assay buffer (Hanks' balanced salt solution, 20 mM HEPES, and 2.5 mM probenecid) for 1 h at 37°C. Dye was removed, and 20 μl of assay buffer was added. Ca2+ flux was measured using the Functional Drug Screening System (FDSS6000; Hamamatsu Corporation, Bridgewater, NJ). Compounds were diluted into assay buffer to a 5× stock that was applied to the cells. For neutral measurements, cells were preincubated with the test compounds for 1 min, potentiator or antagonist was added, and the cells were incubated for an additional 5 min. Cells were then stimulated for 2 min with an appropriate concentration of glutamate. For potentiator and antagonist measurements, cells were preincubated with the test compounds for 5 min and then stimulated for 2 min with an appropriate concentration of glutamate. Data were collected at 0.25 Hz during the preincubation period and at 1 Hz during the glutamate addition phase of the experiment.
Raw data were normalized in a three-step process: 1) Cell number and nonuniform illumination/imaging were controlled for based on the initial readings for the well, 2) the signal amplitude for the data point immediately preceding the agonist addition was subtracted from each point on the trace, and 3) data were normalized to the maximal response for each experiment. Concentration-response curves were generated using Prism 4.0 (GraphPad Software Inc., San Diego, CA).
Radioligand Binding Assays. The allosteric antagonist MPEP analog [3H]methoxyPEPy (Cosford et al., 2003) was used to evaluate the interaction of the test compounds with the allosteric MPEP site on mGlu5. Membranes were prepared from HEK293A cells stably expressing rat mGlu5. Compounds were diluted into assay buffer (50 mM Tris and 0.9% NaCl, pH 7.4) to a 5× stock, and 20 μl of test compound was added to each well of a 96-well assay plate. Sixty-microliter aliquots of membranes diluted in assay buffer (10 μg/well) were added to each well. Twenty microliters of [3H]methoxyPEPy (2 nM final concentration in assay buffer) was added, and the reaction was incubated at room temperature for 60 min with shaking. After the incubation period, the membrane-bound ligand was separated from free ligand by filtration through glass fiber 96-well filter plates (Unifilter-96, GF/B; PerkinElmer Life and Analytical Sciences, Boston, MA). The contents of each well were transferred simultaneously to the filter plate and washed four times with assay buffer (Brandel cell harvester; Brandel Inc., Gaithersburg, MD). Thirty microliters of scintillation fluid was added to each well, and the membrane-bound radioactivity was determined by scintillation counting (TopCount; PerkinElmer Life and Analytical Sciences). Nonspecific binding was estimated using 5 μM MPEP.
Electrophysiology in Subthalamic Nucleus Neurons. Midbrain slices were prepared from 12- to 15-day-old Sprague-Dawley rats as described previously (Awad et al., 2000; Marino et al., 2001). After decapitation, brains were rapidly removed and submerged in an ice-cold choline replacement solution containing 126 mM choline chloride, 2.5 mM KCl, 1.2 mM NaH2PO4, 1.3 mM MgCl2, 8 mM MgSO4, 10 mM glucose, and 26 mM NaHCO3, equilibrated with 95% O2, 5% CO2. The brain was glued to the chuck of a vibrating blade microtome (Leica Microsystems Nussloch GmbH, Nussloch, Germany), and 350-μm-thick slices were obtained. Slices were transferred to a holding chamber containing normal artificial cerebrospinal fluid (ACSF): 124 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 1.0 mM NaH2PO4, 2 mM CaCl2, 20 mM glucose, and 26 mM NaHCO3, equilibrated with 95% O2, 5% CO2 that was maintained at room temperature. In all experiments, 5 μM glutathione, 500 μM pyruvate, and 250 μM kynurenic acid were included in the choline chloride buffer and in the holding chamber ACSF to increase slice viability.
Slices were transferred to the stage of a brain slice chamber and continually perfused with ACSF (∼3 ml/min). Neurons in the subthalamic nucleus (STN) were visualized by the 40× water immersion lens with Hoffman modulation contrast microscope. Patch electrodes were pulled from borosilicate glass on a Narishige (East Meadow, NY) vertical patch pipette puller and filled with internal solution: 125 mM potassium gluconate, 4 mM NaCl, 6 mM NaH2PO4, 1 mM CaCl2, 2 mM MgSO4, 10 mM BAPTA-tetrapotassium salt, 10 mM HEPES, 2 mM Mg-ATP, and 0.3 mM Na2-GTP; pH adjusted to 7.3 with 0.5 N KOH. Electrode resistance was 3 to 7 MΩ. All whole-cell patch-clamp recordings were performed using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Data were digitized with DigiData 1322A (Molecular Devices), filtered (2 kHz), and acquired by the pClamp 9.2 program (Molecular Devices). After formation of a whole-cell configuration, the recorded neurons were current-clamped to -60 mV. Membrane potentials of STN neurons were recorded, and the response to mGlu5 receptor activation input resistance was monitored. All compounds were added by addition to the perfusion solution.
Results
MPEP Analogs Inhibit the Binding of [3H]MethoxyPEPy to mGlu5 Membranes but Do Not Fully Antagonize mGlu5 Response to Glutamate. We have made a broad range of compounds based on the MPEP scaffold (Alagille et al., 2005a,b; Fig. 1). Most of the compounds we have made in this series fully inhibit activation of mGlu5 by glutamate (Alagille et al., 2005a,b). However, we found that a subtle change in the position of the pyridinyl methyl group of MPEP to form 5MPEP (Fig. 1) results in a compound that is completely inactive in inhibiting mGlu5 receptor responses. It was surprising that such a close structural analog of MPEP had no inhibitory effect on the mGlu5 response to glutamate. Thus, we synthesized two related compounds, M-5MPEP and Br-5MPEPy, derived from the potent mGluR5 antagonists M-MPEP and Br-MPEPy (Alagille et al., 2005b) containing similar structural changes, to determine whether this lack of effect was unique to 5MPEP or whether changing the position of this methyl group would also impact activity of related compounds. In addition, the previous studies were performed in Chinese hamster ovary cells that had been transfected with mGlu5, and we wanted to verify that 5MPEP is inactive as an mGlu5 receptor antagonist in a native system. To accomplish this, we tested 5MPEP, M-5MPEP, and Br-5MPEPy for their ability to inhibit mGlu5 receptor-mediated calcium transients in cortical astrocytes. Cortical astrocytes were chosen for these studies because this provides a native system that endogenously expresses high levels of mGlu5 but not of other mGlu receptor subtypes (Peavy et al., 2001, 2002). Consistent with this, the mGlu receptor agonist glutamate induced a robust calcium mobilization response in these cells, and this response was fully blocked by 10 μM MPEP (Fig. 2). Consistent with previous studies in a recombinant system, 5MPEP (10 μM) had no inhibitory effect on the mGlu5 response to glutamate. M-5MPEP and Br-5MPEPy partially inhibited glutamate-induced calcium transients in these cells (Fig. 2).
The most obvious explanation for the lack of activity of 5MPEP in inhibiting mGlu5 is that this structural change reduces affinity for the MPEP site on this receptor so that 10 μM does not bind to mGlu5. Likewise, a plausible explanation for the relatively small effect of single concentrations of M-5MPEP and Br-5MPEPy is that the affinities of these compounds are drastically reduced so that 10 μM only partially occupies the MPEP site. However, it is unusual for such a subtle structural change to have such a drastic effect on affinity of a ligand for its site. Thus, we determined the affinities of each of these compounds at the allosteric antagonist site for MPEP by measuring their ability to displace binding of a close analog of MPEP, [3H]methoxyPEPy (Cosford et al., 2003), to membranes prepared from HEK293A cells stably expressing rat mGlu5 (Fig. 3). Interestingly, all three analogs inhibited the binding of the MPEP analog to mGlu5 membranes at concentrations in the high nanomolar range. The binding affinities of M-5MPEP and Br-5MPEPy (Ki = 145 ± 60 and 182 ± 50 nM) were slightly higher than that of 5MPEP (Ki = 388 ± 48 nM), whereas all three compounds bound to the receptor binding site with a lower affinity than MPEP (Ki = 4.72 ± 1.50 nM). MPEP (5 μM) was used to estimate nonspecific binding (8% of total binding).
Chemical structures of allosteric ligands of mGlu5.
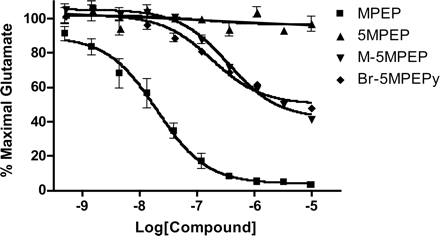
We next determined the effects of a wide range of each of the MPEP analogs on mGlu5 receptor-mediated increases in intracellular calcium (Fig. 4). 5MPEP was inactive as an antagonist of mGlu5 across a range of concentrations that should span the occupancy range for this compound at the MPEP site. Interestingly, M-5MPEP and Br-5MPEPy displayed partial antagonist activity and induced a maximal reduction of the response to glutamate of approximately 50%. The IC50 values of these compounds at inhibiting mGlu5 receptor responses were approximately 300 nM, which is consistent with their affinities at the [3H]methoxyPEPy site.
MPEP analogs do not fully antagonize the mGlu5 receptor response to glutamate in rat cortical astrocytes. A, representative traces show mGlu5 response induced by glutamate in the presence of MPEP and MPEP analogs. Compounds (10 μM) were added to cells loaded with a calcium-sensitive dye and incubated for 5 min. A nearly maximal concentration of glutamate was added, and the calcium response was measured by the FDSS plate reader. B, bar graph illustrates the means of three independent experiments plotted as a percentage of the maximum response to glutamate. Error bars represent S.E.M.
MPEP analogs inhibit the binding of [3H]methoxyPEPy to membranes from cells expressing mGlu5. Membranes prepared from rat mGlu5 HEK293A cells (10 μg/well) were incubated with radiolabeled MPEP analog [3H]methoxyPEPy (2 nM) in the presence of varying concentrations of MPEP analogs for 60 min at room temperature. Samples were filtered through glass fiber filters and washed extensively. Nonspecific binding was estimated with 5 μM MPEP. Concentration-response curves were generated from the means of three separate experiments. Error bars represent S.E.M.
5MPEP Does Not Alter the mGlu5 Concentration-Response Relationship to Glutamate. MPEP and other full allosteric mGlu5 receptor antagonists induce an insurmountable inhibition of the mGlu5 glutamate concentration-response curve and completely inhibit the response to glutamate. Thus, MPEP shifts the glutamate concentration-response curve to the right and downward. In contrast, allosteric potentiators of mGlu5 induce a parallel shift of the glutamate concentration-response curve to the left (O'Brien et al., 2003, 2004; Lindsley et al., 2004; Kinney et al., 2005). Based on this and the finding that M-5MPEP and Br-5MPEPy are partial antagonists of mGlu5, we predicted that they would be expected to shift the glutamate concentration-response curve to the right and decrease in maximal response. However, they would not be expected to flatten the concentration-response relationship of glutamate as is the case for MPEP. Consistent with this prediction, maximally effective concentrations of M-5MPEP and Br-5MPEPy induced a rightward and downward shift of the glutamate concentration-response curve, suggesting they behave as partial noncompetitive antagonists (Fig. 5).
The finding that 5MPEP does not block the response to a near maximal concentration of glutamate suggests that this compound should not shift the glutamate concentration-response curve to the right. However, it is possible that 5MPEP could act as an allosteric potentiator and shift the curve to the left. Interestingly, 5MPEP had no effect on the glutamate concentration-response curve, suggesting that it is neither an allosteric antagonist nor potentiator of mGlu5.
5MPEP Blocks MPEP Inhibition of mGlu5-Induced Calcium Mobilization. The finding that 5MPEP binds to the MPEP site but does not alter the glutamate concentration-response relationship suggests that this compound must be a neutral or silent ligand at this site. If this is the case, 5MPEP would be expected to competitively block the allosteric antagonist response to MPEP. Based on this hypothesis, we predicted that 5MPEP would bind to the MPEP site of mGlu5 and compete with MPEP, thereby blocking the inhibitory effects of the allosteric antagonist. In a similar manner, if M-5MPEP and Br-5MPEPy are partial antagonists at this site, they should also compete with MPEP for binding and block its effects. However, because M-5MPEP and Br-5MPEPy partially inhibit the response to glutamate, they should only partially block the inhibitory response to MPEP. Figure 6 shows the effects of multiple concentrations of each of these compounds on the calcium mobilization response to glutamate when added alone and in the presence of MPEP. MPEP (50 nM) induces a robust inhibition of the calcium response of mGlu5 to a nearly maximal concentration of glutamate. We determined the concentration-response relationship of modulators 5MPEP, M-5MPEP, and Br-5MPEPy for blocking the inhibitory activity of MPEP on the glutamate response. For comparison, the concentration-response relationship of each compound on the response to glutamate alone is shown. As predicted, each compound induced a concentration-dependent reduction in MPEP-induced inhibition of mGlu5. M-5MPEP and Br-5MPEPy only partially blocked the response to MPEP to the level observed with each compound alone. In contrast, 5MPEP fully blocked the response to MPEP in a concentration-dependent manner with an EC50 = 2.32 ± 0.21 μM. As we hypothesized, 5MPEP acts as a neutral allosteric ligand by having no impact on the mGlu5 response alone but being capable of blocking the effects of the antagonist MPEP.
5MPEP does not antagonize the mGlu5 receptor response to glutamate in rat cortical astrocytes. Varying concentrations of MPEP and MPEP analogs were added to calcium-sensitive dye-loaded cells and incubated for 5 min. A nearly maximal concentration of glutamate was added, and the calcium response was measured by the FDSS plate reader. Concentration-response curves were generated from the mean data of three experiments. Data are plotted as a percentage of the maximum response to glutamate. Error bars represent S.E.M.
5MPEP Blocks MPEP Inhibition of mGlu5 Activity in a Competitive Manner. Our binding data indicate 5MPEP competes with [3H]methoxyPEPy for the MPEP binding site and our functional data demonstrate 5MPEP blocks the inhibitory effects of MPEP on mGlu5 activity. If 5MPEP blocks MPEP action by competitive interaction with a single allosteric site, 5MPEP should induce a parallel shift in the MPEP concentration-response curve to the right. Figure 7 shows the effects of increasing concentrations of 5MPEP (3, 10, and 30 μM) on the MPEP concentration-response relationship. As can be seen, each concentration of 5MPEP induced a parallel rightward shift in the MPEP concentration-response curve. Furthermore, a Schild analysis of the effects of 5MPEP on the MPEP concentration response yielded a linear regression with a slope of 1.076 ± 0.01 (r2 = 0.99), indicating the relationship between 5MPEP and MPEP to be competitive. Extrapolation of the line to the x-intercept established a Ki of ∼530 nM, a result consistent with the Ki value for 5MPEP at the [3H]methoxyPEPy site as determined by radioligand binding. The Schild regression data combined with radioligand binding data lead us to conclude that 5MPEP binds to the MPEP site and blocks MPEP inhibition of mGlu5-induced calcium mobilization in a competitive manner.
5MPEP does not alter the concentration-response relationship of mGlu5 to glutamate. MPEP analogs (10 μM) were added to calcium-sensitive dye-loaded cells and incubated for 5 min. A range of glutamate concentrations was added, and the calcium response was measured by the FDSS plate reader. Concentration-response curves were generated from the mean data of at least three experiments. Data are plotted as a percentage of the maximum response to 100 μM glutamate. Error bars represent S.E.M.
5MPEP Blocks Potentiation of mGlu5-Induced Calcium Mobilization by Multiple Structural Classes of Potentiators. DFB and CDPPB are allosteric potentiators of mGlu5 derived from two different structural scaffolds, both of which are distinct from the MPEP scaffold (Fig. 8A). However, both of these compounds displace [3H]methoxyPEPy binding, leading to the hypothesis that their allosteric potentiator activity is mediated by binding to the same allosteric site as that of MPEP (O'Brien et al., 2003; Lindsley et al., 2004; Kinney et al., 2005). If this is the case, then the neutral allosteric site ligand 5MPEP should inhibit the allosteric potentiator responses to DFB and CDPPB. Consistent with previous reports in recombinant systems, mGlu5-mediated increases in intracellular calcium in response to a suboptimal concentration of glutamate were potentiated by DFB and CDPPB in rat cortical astrocytes (Fig. 8B). Concentrations of DFB (30 μM) and CDPPB (3 μM) used for this experiment were chosen based on a minimum concentration of each required to elicit a maximal potentiator response. 5MPEP (30 μM) does not alter the mGlu5 response to glutamate alone, but it completely blocks the allosteric potentiator responses to both DFB and CDPPB (Fig. 8B). We then determined the concentration-response relationship of 5MPEP for blocking the potentiator activity of CDPPB, the more potent potentiator of the two classes, on the glutamate response relationship (Fig. 8C). Inhibition of the potentiator response was found to be concentration-dependent, blocking the response with an IC50 = 1.71 ± 0.32 μM. This is consistent with the concentration-response relationship of 5MPEP at blocking the response to MPEP (Fig. 6). For comparison, the affinity values calculated for 5MPEP, M-5MPEP, and Br-5MPEPy determined by radioligand binding and calculated from the EC50 of the inhibition of the effect of MPEP (Fig. 6), as well as the affinity values for 5MPEP calculated from the Schild analysis (Fig. 7) and the IC50 of the inhibition of the effect of CDPPB (Fig. 8C), are shown in Table 1.
Binding affinity of MPEP analogs calculated from displacement of [3H]methoxyPEPy, Schild analysis, inhibition of the effect of MPEP, and inhibition of the effect of CDPPB Values are expressed as mean ± S.E.M.
5MPEP reduces allosteric inhibition of mGlu5 by MPEP in a concentration-dependent manner. Varying concentrations of 5MPEP (A), M-5MPEP (B), and Br-5MPEPy (C) were added to calcium-sensitive dye-loaded cells followed 1 min later by addition of a single concentration of MPEP (50 nM). After a 5-min incubation, a nearly maximal concentration of glutamate was added, and the calcium response was measured by the FDSS plate reader. For comparison, the concentration-response relationship of each compound on the response to glutamate alone is shown. Concentration-response curves were generated from the mean data of three experiments. Data are plotted as a percentage of the maximum response to glutamate. Error bars represent S.E.M.
5MPEP reduces allosteric inhibition by MPEP in a competitive manner. A, multiple concentrations of 5MPEP (3, 10, or 30 μM) were added to calcium-sensitive dye-loaded cells followed 1 min later by addition of varying concentrations of MPEP. After a 5-min incubation, a nearly maximal concentration of glutamate was added, and the calcium response was measured by the FDSS plate reader. Concentration-response curves were generated from the mean data of three experiments. Data are plotted as a percentage of the maximum response to glutamate. Error bars represent S.E.M. B, Schild analysis of results indicates inhibition of MPEP by 5MPEP is competitive (slope, 1.076 ± 0.009; x-intercept, -6.25).
A, DFB and CDPPB, two structurally distinct classes of mGlu5 potentiators. B, 5MPEP reduces allosteric potentiation of mGlu5 response by DFB and CDPPB. Either CDPPB (3 μM) or DFB (30 μM) was added to calcium-sensitive dye-loaded cells and incubated for 5 min. A suboptimal concentration of glutamate was added, and the calcium response was measured by the FDSS plate reader. For neutral ligand experiments, 5MPEP (30 μM) was added to calcium-sensitive dye-loaded cells followed 1 min later by addition of a single concentration of CDPPB (3 μM) or DFB (30 μM). After a 5-min incubation, a suboptimal concentration of glutamate was added, and the calcium response was measured by the FDSS plate reader. The bar graph represents the mean data of three experiments. Data are plotted as a percentage of the maximum response to 100 μM glutamate. C, 5MPEP reduces allosteric potentiation of mGlu5 response by CDPPB in a concentration-dependent manner. Experiments were performed as described in B. The concentration-response curve was generated from the mean data of four experiments. Data are plotted as a percentage of the maximum potentiation of a suboptimal glutamate response. Error bars represent S.E.M.
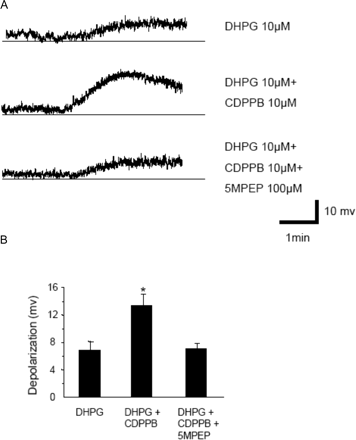
5MPEP Blocks Effects of CDPPB and MPEP on mGlu5 Receptor Responses in STN Neurons. Discovery of 5MPEP as a neutral ligand with submicromolar affinity at the MPEP site on mGlu5 suggests that this compound could provide a valuable tool for evaluating functional responses to allosteric mGlu5 receptor antagonists and potentiators. Thus, any responses to MPEP or CDPPB that are mediated by actions of these compounds on mGlu5 should be blocked by 5MPEP. We previously found that MPEP blocks DHPG-induced depolarization of neurons in the STN, leading us to suggest that mGlu5 is important for depolarization of these neurons (Awad et al., 2000). Thus, we determined the effect of 5MPEP on STN neurons to determine whether this compound has effects in these cells that are consistent with neutral allosteric site activity. Whole-cell recordings were performed from STN neurons in rat midbrain slices in the presence of tetrodotoxin (500 nM), which blocks action potential firing. As reported previously (Awad et al., 2000), bath application of DHPG (100 μM) induced a robust depolarization in these cells (14.82 ± 2.21 mV; n = 8) (Fig. 9, A and B). In addition, consistent with our previous report, the DHPG-induced response was inhibited by the mGlu5 receptor-selective antagonist MPEP (10 μM) (6.15 ± 0.82 mV; n = 12) (Fig. 9, A and B), suggesting that it is mediated by activation of mGlu5. Interestingly, 5MPEP had no effect on membrane potential of STN neurons (Fig. 9A). However, preincubation with 5MPEP (100 μM) for 10 min completely blocked the inhibitory effect of MPEP on DHPG-induced response (14.28 ± 2.1mV; n = 9) (Fig. 9, A and B).
We next determined the effect of the allosteric potentiator CDPPB on the response to a lower concentration of DHPG (10 μM) that induces a submaximal depolarization of STN neurons (6.89 ± 1.22 mV; n = 6) (Fig. 10, A and B). Consistent with the allosteric potentiator effects of CDPPB on mGlu5 in recombinant systems and cortical astrocytes, CDPPB (10 μM) induced a robust potentiation of depolarization of STN neurons by DHPG (10 μM) (13.39 ± 1.66 mV; n = 8) (Fig. 10, A and B). Furthermore, the potentiator response to CDPPB was completely blocked by preincubation with 100 μM 5MPEP (7.05 ± 0.82 mV; n = 10). These data provide convincing evidence that CDPPB potentiates depolarization responses to mGlu5 receptor activation in STN neurons and indicate that 5MPEP acts as a neutral ligand of mGluR5 in both cortical astrocytes and STN neurons.
Discussion
This report describes the characterization of 5MPEP as a novel neutral allosteric site ligand of mGlu5. This compound interacts with the allosteric site on mGlu5 in a manner that is directly analogous to that of a neutral antagonist at an orthosteric neurotransmitter binding site. This is also similar to activity that we recently reported for 3,3′-dichlorobenzaldazine, an analog of DFB that acts as a neutral ligand at this site (O'Brien et al., 2003). However, unlike 5MPEP, 3,3′-dichlorobenzaldazine did not have sufficient potency and solubility to allow rigorous characterization or use in more complex systems such as brain slice preparations that we now report for 5MPEP. In addition, we have identified M-5MPEP and Br-5MPEPy as two novel compounds that act as partial antagonists at the same allosteric site on mGlu5. Although these compounds have some similarities to partial agonists at orthosteric sites, they have actions at a functional level that are fundamentally different from those of partial agonists.
5MPEP inhibits the effects of MPEP on mGlu5 receptor responses in STN neurons. A, representative traces show depolarization of STN neurons by application of DHPG (100 μM), effect of MPEP (10 μM) on DHPG-induced depolarization, and DHPG-induced depolarization of STN neurons in the presence of MPEP (10 μM) and 5MPEP (100 μM). B, bar graph (mean ± S.E.M.) illustrates depolarization of STN neurons by DHPG (n = 8 cells), DHPG plus MPEP (n = 12 cells), and DHPG in the presence of 5MPEP plus MPEP (n = 9 cells). *, p < 0.01; Student's t test.
Neutral antagonists at orthosteric sites of GPCRs are similar to 5MPEP in that they bind silently to the orthosteric site, and they do not activate or decrease constitutive activity of the receptor. However, neutral orthosteric site ligands block the effects of agonists or inverse agonists. Extension of this concept to allosteric sites on GPCRs and the use of 5MPEP to characterize the pharmacological properties of this allosteric site on mGlu5 provide important insight into the nature of this allosteric site and suggest that ligand interactions with the allosteric site on the mGlu5 receptor follow the same rules of traditional receptor theory that were established with ligand interactions at orthosteric binding sites. Thus, 5MPEP binds to mGlu5 but neither potentiates nor inhibits the response to glutamate when added alone. However, 5MPEP blocks the allosteric antagonist activity of MPEP and the allosteric potentiator activity of CDPPB and DFB. Furthermore, analysis of the effect of 5MPEP on the MPEP concentration-response relationship reveals that these ligands regulate functional responses of the receptor in a competitive manner. Thus, analysis of these data using a Schild analysis (Arunlakshana and Schild, 1959) suggests a competitive interaction and provides an estimate of the Ki value of the neutral ligand that is consistent with that determined by measuring displacement of a radiolabeled ligand to the allosteric site. Although analogous to neutral orthosteric ligands, neutral ligands at an allosteric site are fundamentally different in that they are not receptor antagonists. Thus, they do not inhibit the response of mGlu5 to glutamate.
5MPEP inhibits the effects of CDPPB on mGlu5 receptor responses in STN neurons. A, representative traces show depolarization of STN neurons induced by DHPG (10 μM), potentiation effect of CDPPB (10 μM) on DHPG-induced depolarization, and DHPG-induced depolarization of STN neurons in the presence of CDPPB (10 μM) and 5MPEP (100 μM). B, bar graph (mean ± S.E.M.) illustrates depolarization of STN neurons by DHPG (n = 6 cells), DHPG plus CDPPB (n = 8 cells), and DHPG in the presence of 5MPEP plus CDPPB (n = 10 cells). *, p < 0.01; Student's t test.
In addition to providing insight into the pharmacological properties of allosteric sites on GPCRs, discovery of 5MPEP expands the toolbox of valuable ligands available to us to study the physiological significance of allosteric modulation of GPCRs. Although marked progress in the field of allosteric modulation of mGlu receptors and other GPCRs has recently been made, a true understanding of the mechanism by which these ligands regulate receptor function has yet to be established. In addition, we have few tools that allow us to assess the effects of allosteric agonists and antagonists in native systems. Allosteric potentiators and antagonists such as CDPPB and MPEP are playing a central role in developing an understanding of the functional roles of mGlu5 and other GPCRs. By selectively blocking the effects of a potentiator or antagonist but having no effects on the targeted receptor itself, neutral allosteric ligands such as 5MPEP provide exciting new tools that make it possible to evaluate whether a functional response to an allosteric antagonist or potentiator is actually mediated by the targeted receptor. Continued development of neutral ligands will have a major impact on the forward progress of in vivo studies of allosteric modulators of GPCRs and hence on the understanding of their mechanism of action.
In addition to blocking effects of exogenously applied or administered allosteric potentiators or antagonists, 5MPEP could provide an exciting tool to aid in studies aimed at determining whether endogenous ligands exist for the allosteric site on mGlu5. Although tremendous progress has been made in identifying synthetic compounds that act at allosteric sites on GPCRs, the question as to whether endogenous ligands for these sites remains open. Because 5MPEP is inactive in the absence of an allosteric potentiator or antagonist, it will be of interest to determine whether physiological effects of this compound can be observed under some settings. In the present study, we found that 5MPEP has no effect on mGlu5 in astrocytes or STN neurons when added alone. In future studies, it will be important to determine whether this compound has activity on responses to afferent stimulation or in other settings that may favor release of an endogenous ligand at the allosteric site. If 5MPEP is found to have effects on mGlu5 when added alone, this would provide indirect evidence that an endogenous ligand may exist and provide insights into the physiological conditions that could favor their synthesis or release.
Discovery of partial antagonists of mGlu5 also has important implications for the range of activity possible for allosteric site ligands and the potential utility of compounds that interact with the allosteric site. As mentioned above, partial antagonist activity of M-5MPEP and Br-5MPEPy is in some ways analogous to the activity of orthosteric site partial agonists. For both partial antagonists and partial agonists, these compounds will partially block the response of a GPCR to its natural orthosteric ligand. However, unlike partial agonists, M-5MPEP and Br-5MPEPy do not partially activate mGlu5 when added alone. Furthermore, these compounds only partially inhibit the receptor. Such partial antagonists could be useful in settings where there is a need to maintain some level of receptor activity but to inhibit effects of excessive receptor activation. This partial antagonist activity is unique to the novel compounds described here and could only be achieved with allosteric site ligands. Thus, this illustrates another important property of ligands at allosteric sites on GPCRs that could not be achieved with orthosteric site ligands and that could be useful in discovery of novel therapeutic agents.
A final important point is that previous studies of action of allosteric potentiators of mGlu5 have been largely restricted to studies in cell lines in which mGlu5 is overexpressed. The present findings in cortical astrocytes and STN neurons suggest that allosteric potentiators of mGlu5 have effects in these native systems that are virtually identical in nature to those described previously in recombinant systems. Furthermore, inhibition of these responses by 5MPEP, which is structurally dissimilar from either DFB or CDPPB, provides strong evidence that these effects of the allosteric potentiators are mediated by mGlu5. These exciting data provide strong support for the potential utility of allosteric potentiators of mGlu5 for increasing activity of this receptor in a range of cells that natively express this receptor.
Footnotes
-
This work was supported by grants from National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Institute on Drug Abuse, the Stanley Foundation, and National Alliance for Research on Schizophrenia and Depression.
-
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
-
doi:10.1124/mol.105.016139.
-
ABBREVIATIONS: mGlu, metabotropic glutamate receptor; GPCR, G protein-coupled receptor; MPEP, 2-methyl-6-(phenylethynyl)-pyridine; DFB, 3,3′-difluorobenzaldazine; CDPPB, 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide; M-5MPEP, 2-(3-methoxyphenylethynyl)-5-methylpyridine; Br-5MPEPy, 3-(6-methylpyridin-2-ylethynyl)-5-bromopyridine; 5MPEP, 5-methyl-2-phenylethynyl-pyridine; methoxy-PEPy, 3-methoxy-5-(2-pyridinylethynyl)pyridine; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; HEK, human embryonic kidney; FDSS, Functional Drug Screening System; ACSF, artificial cerebrospinal fluid; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; STN, subthalamic nucleus; DHPG, 3,5-dihydroxyphenylglycine.
- Received June 29, 2005.
- Accepted September 9, 2005.
- The American Society for Pharmacology and Experimental Therapeutics