Abstract
The endocannabinoid 2-arachidonoylglycerol (2-AG) enhances cell migration through the CB2 cannabinoid receptor. In this study, using an immunoprecipitation and mass spectrometry-based proteomic approach, we first identified the 90-kDa heat shock protein (Hsp90), a chaperone protein with novel signaling functions, as a CB2-interacting protein. The CB2/Hsp90 interaction was confirmed in human embryonic kidney 293 cells expressing transfected CB2 and in differentiated HL-60 cells expressing endogenous CB2, by coimmunoprecipitation and Western blot experiments, as well as by treatment with geldanamycin (GA), a specific Hsp90 inhibitor. Disruption of the CB2/Hsp90 interaction by treatment with GA or reducing Hsp90 levels with specific short interfering RNAs markedly inhibited 2-AG-induced cell migration, demonstrating that Hsp90 is crucial for 2-AG-induced cell migration. 2-AG treatment resulted in a CB2-mediated stimulation of Rac1 activity, and treatment with GA blocked 2AG-induced activation of Rac1. It is noteworthy that expression of the dominant-negative form of Rac1 reduced 2-AG-induced cell migration. These data demonstrate that 2-AG-induced activation of Rac1 is essential for 2-AG-induced cell migration, and the CB2/Hsp90 interaction is needed for 2-AG-induced activation of Rac1. Furthermore, 2-AG-induced Rac1 activation was sensitive to pertussis toxin treatment, hence involving Gi proteins. In addition, treatment with GA significantly inhibited the CB2/Gαi2 interaction. As a whole, our data indicate that Hsp90 may serve as scaffold to keep the CB2 receptor and its signaling components, including Gαi2, in proximity, thus facilitating CB2-mediated signaling to cell migration through the Gi-Rac1 pathway. By demonstrating that Hsp90 is essential for CB2-mediated signaling to cell migration, this study reveals a novel role of Hsp90 in the signaling events mediated by a G protein-coupled receptor.
G protein-coupled receptors (GPCRs) are of vital importance for cellular functions, and they are important targets for drug discovery (Gao and Jacobson, 2006). Two G protein-coupled receptors have been identified as cannabinoid receptors, and they are referred to as CB1 and CB2 (Matsuda et al., 1990; Munro et al., 1993). CB1 is predominantly expressed in the brain, whereas CB2 is mainly expressed on cells of immune system (Matsuda et al., 1990; Munro et al., 1993; Pertwee, 1997; Buckley et al., 1998). In addition to psychotropic effects mediated by the CB1, many studies have described a cannabinoid-induced modulation of immune functions, which could be attributed to the CB2 (Sugiura et al., 2004; Pertwee, 2005; Buckley et al., 2006; Lunn et al., 2006).
2-Arachidonoylglycerol (2-AG) is an endogenous ligand for the cannabinoid receptor (Mechoulam et al., 1995; Sugiura et al., 1995). 2-AG binds to both CB1 and CB2, and it exhibits a variety of cannabimimetic activities in vitro and in vivo (Mechoulam et al., 1995; Sugiura et al., 1995; Sugiura and Waku, 2000; Kogan and Mechoulam, 2006). Evidence is accumulating that 2-AG is a physiologically essential molecule that acts both as a novel type of neuromodulator and as an important immunomodulator (Sugiura et al., 2004; Kogan and Mechoulam, 2006).
Cell migration is of paramount importance in physiological activities of, for example, leukocyte-mediated immune surveillance, as well as in pathological processes such as leukemia metastasis. Endocannabinoid 2-AG has been reported to enhance the migration of a variety of cell types (e.g., normal leukocytes as well as differentiated and undifferentiated leukemia cells) through the CB2 receptor (Jordà et al., 2002, 2003; Kishimoto et al., 2003; Oka et al., 2004). Despite the important roles that CB2 receptors play in the migration of normal leukocytes and leukemia cells, the identification of molecular mechanisms underlying CB2 receptor-mediated cell migration is still in its early stages.
Recent evidence has strongly suggested that GPCRs interact with a variety of proteins in addition to G proteins, which can participate in the trafficking, signaling, fine-tuning, and allosteric regulation of GPCRs (Brady and Limbird, 2002; Bockaert et al., 2004; Tilakaratne and Sexton, 2005). In this study, to better define the CB2 receptor complex involved in cell migration, we first used a coimmunoprecipitation and mass spectrometry-based proteomic approach to study the CB2 receptor-interacting proteins. We then focused on Hsp90, one of the CB2 receptor-interacting proteins that we identified using the proteomic technology.
Hsp90 is a highly conserved protein. Its role as a molecular chaperone in preventing protein aggregation and in promoting refolding of denatured proteins has been well studied (Rutherford and Zuker, 1994; Bohen et al., 1995; Pratt, 1997; Gaestel, 2006). More recently, interest in Hsp90 has expanded to include an apparently mechanistically distinct function. By forming heterocomplexes with its substrates, Hsp90 has been shown to play a key role as scaffolding site for various signaling events under nonstress conditions (Rutherford and Zuker, 1994; Bohen et al., 1995; Pratt, 1997; Gaestel, 2006).
In this study, we reveal that there are interactions between Hsp90 and CB2 receptors in HEK293 cells expressing transfected CB2 receptors as well in differentiated HL-60 cells expressing endogenous CB2 receptors. Furthermore, we demonstrate that Hsp90 is involved in the CB2 receptor signaling and CB2 receptor-mediated cell migration.
Materials and Methods
Materials. The following primary antibodies were used in our experiments: mouse monoclonal anti-FLAG M2 antibody (Sigma-Aldrich, St. Louis, MO), rabbit polyclonal anti-CB2 antibody (Cayman Chemical, Ann Arbor, MI), mouse monoclonal anti-Hsp90 antibody (BD Biosciences, San Jose, CA), rabbit anti-mouse IgG (Chemicon International, Temecula, CA), His-Tag monoclonal antibody (EMD Biosciences, Madison, WI), mouse monoclonal anti-hemagglutinin antibody (Roche Diagnostics, Indianapolis, IN), rabbit polyclonal anti-β-actin antibody (Cell Signaling Technology Inc., Danvers, MA), and rabbit polyclonal anti-Rac antibodies (Cytoskeleton, Denver, CO). The horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit secondary antibodies were purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). 2-AG was obtained from Tocris Cookson Inc. (Ellisville, MO). SR144528 was from National Institute of Drug Abuse (Bethesda, MD). Geldanamycin was from BIOMOL Research Laboratories (Plymouth Meeting, PA). pIRESneo vector was from BD Biosciences. The plasmid encoding human dominant-negative Rac-1 proteins was obtained from University of Missouri-Rolla cDNA Resource Center (Rolla, MO). Hsp90 siRNA and scrambled siRNA were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). DMEM, RPMI 1640 medium, and EDTA-trypsin were from Thermo Fisher Scientific (Waltham, MA). Lipofectamine 2000 was from Invitrogen (Carlsbad, CA). HEK293 and HL-60 cells were from American Type Culture Collection (Manassas, VA).
Cell Culture. Transfected HEK293 cells were grown in DMEM supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 20 mM l-glutamine, and 500 μg/ml G-418 (Geneticin; Invitrogen). HL-60 promyelocytic leukemia cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 20 mM l-glutamine. Cultures were maintained at 37°C in an atmosphere of 95% air and 5% CO2. To differentiate HL-60 cells into neutrophil-like cells, 250 μl of DMSO was added to 2 × 106 cells in 20 ml of RPMI 1640 medium, followed by an incubation of 5 days at 37°C in the CO2 incubator without changing the medium.
DNA Constructs and Transfection. To facilitate coimmunoprecipitation, a FLAG sequence was inserted into the N-terminal of human CB2 receptor contained in mammalian expression vector pIRESneo. Sixteen micrograms of plasmid was transfected into HEK293 cells at a confluence of 80% in 10-cm culture plate using Lipofectamine 2000. Transfected cells were selected in culture medium containing 500 μg/ml G-418, and cell lines stably expressing FLAG-CB2 receptor were established according to a method developed previously (Song and Bonner, 1996; Song et al., 1999). In siRNA knockdown experiments, 2 μg of Hsp90 siRNA or scrambled siRNA was transiently transfected into HEK293 cells stably expressing FLAG-CB2 receptor at a confluence of 80% or differentiated HL-60 cells at a density of 2 × 106 cells/ml, using Lipofectamine 2000. Transient transfection of cells with the dominant-negative Rac1 plasmid (16 μg) was performed using Lipofectamine 2000, according to the manufacturer's instructions. Subsequent assays were performed at 24 h after transfection.
Membrane Preparation and Solubilization. For membrane preparations, cells were washed twice with ice-cold phosphate-buffered saline, scraped off the tissue culture plates, and collected by centrifugation at 1000g for 5 min at 4°C. Subsequently, the cells were homogenized in membrane buffer (50 mM Tris-HCl, 5 mM MgCl2, and 2.5 mM EDTA, pH 7.4) with a Polytron homogenizer (Kinematica, Basel, Switzerland). After the homogenate was centrifuged at 32,000g for 20 min at 4°C, the pellet was resuspended in membrane buffer containing 1% dodecylmaltoside, and the sample was incubated with rotation for 2 h at 4°C. The solubilized samples were then centrifuged for 20 min at 100,000g for 20 min at 4°C, and the protein content in the supernatant was determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA).
Immunoprecipitation. For coimmunoprecipitation experiments, 500 μg of proteins from solubilized membrane preparations was incubated with 20 μg of primary antibody and 80 μl of protein G-Sepharose beads. After overnight incubation at 4°C, the resins were washed with 10 volumes of solubilization buffer with increasing concentrations of NaCl, and proteins were eluted from beads in sample loading buffer.
In-Gel Digestion and MALDI-TOF-MS. Samples containing immunoprecipitated receptor complex were separated by SDS-PAGE. The Coomassie-stained gel pieces were destained by three cycles of dehydration with 50% acetonitrile (ACN) in 25 mM NH4HCO3, pH 8.4, and hydration with 25 mM NH4HCO3. The destained protein bands were dried in a vacuum centrifuge. The protein bands were reduced with 10 mM dithiothreitol in 25 mM NH4HCO3 for 45 min at 56°C. For alkylation of proteins, the dithiothreitol solution was replaced by 55 mM iodoacetic acid in 25 mM NH4HCO3 and incubated for 30 min in the dark. The gel pieces were then washed alternatively with 25 mM NH4HCO3 and 25 mM NH4HCO3/50% ACN three times, and they were completely dried in a vacuum centrifuge. The gel pieces were incubated with trypsin in 25 mM NH4HCO3 at 37°C for 16 h. The digestion peptide fragments were mixed with equal amount of matrix solution (α-cyano-cinnamic acid in 50% ACN/0.1% trifluoroacetic acid), and loaded onto target plate. Mass spectra were obtained using a TofSpec 2E MALDI-TOF mass spectrometer (Micromass, Manchester, UK). Proteins were identified based on matching the MS data with the calculated mass values of peptide fragments using Mascot (Matrix Science, London, UK).
Western Blot Analysis. Samples were incubated with 2× Laemmli buffer under reducing conditions at room temperature for 20 min, and proteins were resolved on a 10% SDS-polyacrylamide gel using a minigel electrophoresis system (Invitrogen). Proteins were transferred onto a nitrocellulose membrane for immunodetection with indicated antibodies. The nitrocellulose membranes were blocked with 5% nonfat dry milk in Tris-buffered saline/Tween 20 (TBS-T; 20 mM Tris, 137 mM NaCl, and 0.1% Tween 20, pH 7.6) for 1 h and then incubated overnight at 4°C with the primary antibody. Thereafter, the membranes were washed twice for 10 min each time with TBS-T buffer and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The membranes were then washed three times with TBS-T buffer for 10 min each time, and the antibody-recognized protein bands were visualized using an enhanced chemiluminescence kit from GE Healthcare.
Migration Assay. The migration of differentiated HL-60 cells was assayed using the Transwell inserts (5-μm pore size) and 24-well culture plates (Costar; Corning Life Science, Acton, MA). In brief, 2-AG or vehicle (0.01% DMSO in RPMI 1640 medium) was added to 0.6 ml of the RPMI 1640 medium containing 0.1% bovine serum albumin in the well of the culture plate (the bottom compartment). Subsequently, 1.0 × 106 cells were washed several times, suspended in 0.1 ml of RPMI 1640 medium containing 0.1% bovine serum albumin, and transferred to the Transwell insert (the top compartment). After incubation at 37°C for 4 h in a cell culture incubator, the number of cells that migrated from the top to the bottom compartment was counted using a hemocytometer. For adherent transfected HEK293 cells, Transwell inserts (8-μm pore size) were first coated overnight at 4°C with 5 μg/ml fibronectin. Cells were harvested from the culture dish into a 50-ml centrifuge tube using phosphate-buffered saline-EDTA. Subsequently, the cells were washed with DMEM supplemented with 0.2% bovine serum albumin and centrifuged at 500g. The cell pellets were resuspended in the same medium with a final cell concentration of 1.0 × 106 cell/ml. 2-AG or vehicle (0.01% DMSO in DMEM) was added to 0.6 ml of DMEM supplemented with 0.2% bovine serum albumin in the well of the culture plate (the bottom compartment). The 0.1 ml of transfected HEK293 cells (containing 1.0 × 105 cells) were transferred to the Transwell inserts (the top compartment). After incubation at 37°C for 4 h in cell culture incubator, the Transwell insert was stained with a HEMA 3 staining kit (Fisher Scientific Inc., Houston, TX). Five randomly chosen microscopy fields were photographed. Migrated cells in each filed were quantified by counting using a scorer and the Northern Eclipse software (Empix Imaging Inc. Mississauga, ON, Canada). Results are presented as migration index, which is the ratio of cells migrated in the presence of drug versus cells migrated in the presence of vehicle. Three independent experiments were performed. In each experiment, triplicate was performed for each condition.
Rac1-GTPase Pull-Down Assay. Rac1 activation assay kit was purchased from Cytoskeleton, and assays were performed according to attached protocols. In brief, transfected HEK293 cells with a confluent of 90% in 10-cm culture plate or 1.0 × 107 differentiated HL-60 cells were serum-starved for 18 h. Then, the cells (pretreated with geldanamycin or vehicle: 0.01% DMSO in DMEM or RPMI 1640 medium) were stimulated with 500 nM 2-AG or vehicle (0.01% DMSO in DMEM or RPMI 1640 medium) and immediately lysed with lysis buffer supplied in the kit and centrifuged at 14,000g for 3 min. A portion of each supernatant was diluted in SDS-PAGE sample buffer for detection of total (both GTP- and GDP-bound) Rac1 GTPases. The remaining supernatant fractions were incubated with 20 μl of PAK-PBD-agarose for 1 h at 4°C, followed by washing twice with lysis buffer and wash buffer. Proteins bound to the beads were eluted in 30 μlof2×SDS-PAGE sample buffer, and they were subjected to Western blot analysis.
Data Analysis. The data presented in figures represent mean ± S.E. The figures were generated with the use of GraphPad Prism software (GraphPad Software Inc., San Diego, CA). One-way ANOVA with Newman-Keuls post test was used to compare the data of different treatment groups. The level of significance was chosen as P < 0.01.
Results
Identification of Hsp90 as a CB2 Receptor-Interacting Protein by Proteomic Analysis. A proteomic strategy combining immunoprecipitation and mass spectrometry was used in this study to identify CB2 receptor-interacting proteins. To facilitate the immunoprecipitation of CB2 receptor protein complex, we introduced a FLAG tag sequence at the N-terminal of the human CB2 receptor. In a previous study, we have shown that the FLAG tag sequence does not affect the functions of human CB2 cannabinoid receptor (Zhang et al., 2007).
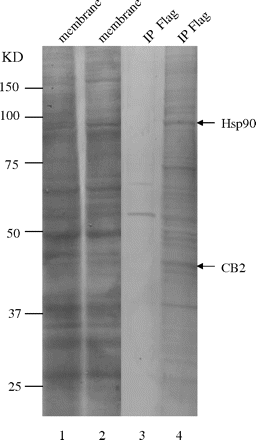
Using an anti-FLAG antibody, CB2 receptor complex was immunoprecipitated from HEK293 cells stably expressing FLAG-CB2. HEK293 cells transfected with an empty vector were used as a negative control, and they were subjected to the same immunoprecipitation procedure. As shown in lane 4 of Fig. 1, when the immunoprecipitated receptor complex was separated by SDS-PAGE and stained with Coomassie Blue, bands ranging in sizes from 37 to 100 kDa were apparent. In contrast, most of these bands were not present in the negative control sample (Fig. 1, lane 3). These experiments were repeated three times, and similar protein bands profiles were obtained. Bands that appeared only in immunoprecipitates of HEK293 cells stably expressing FLAG-CB2 were excised, subjected to trypsin digestion, and identified by MALDI-TOF-MS. Among the protein bands identified unambiguously by MALDI-TOF-MS were CB2 receptor at approximately 40 kDa and Hsp90 at approximately 90 kDa. As shown in Table 1, eight peptides obtained from tryptic digestion of the 90-kDa band matched with calculated molecular mass for the tryptic peptides of Hsp90.
Identification of Hsp90 protein by MALDI-TOF-MS
Comparison of the molecular masses obtained from MALDI-TOF-MS for the tryptic digests of the 90-kDa protein and the calculated molecular masses for the tryptic peptides of Hsp90.
Identification of Hsp90 as a CB2 receptor-interacting protein by proteomic analysis. Protein staining demonstrating immunoprecipitation of the CB2 receptor protein complex from HEK293 cells stably expressing FLAG-CB2. Lanes 1 and 2 represent membrane preparations from HEK293 cells stably transfected with an empty expression vector and HEK293 cells stably expressing FLAG-CB2, respectively. Lanes 3 and 4 represent immunoprecipitates from HEK293 cells stably transfected with an empty expression vector and HEK293 cells stably expressing FLAG-CB2, respectively. The position of CB2 receptor and Hsp90 identified by mass spectrometry is indicated. The experiment was repeated three times, with similar results.
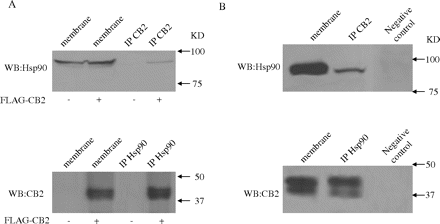
Verification of the Interaction between Hsp90 and CB2 Receptor. The association of Hsp90 with CB2 receptor was confirmed by coimmunoprecipitation and immunoblotting experiments. First, CB2 receptor complex was pulled down from HEK293 cells stably expressing FLAG-CB2 with an anti-CB2 antibody and immunoblotted with the anti-Hsp90 antibody. As shown in Fig. 2A, top, the Hsp90 band was detected in membranes prepared from HEK293 cells stably expressing FLAG-CB2, as well as HEK293 cells transfected with an empty vector. However, after immunoprecipitation, the Hsp90 band was detected only in immunoprecipitates of HEK293 cells expressing FLAG-CB2 receptor and not in HEK293 cells transfected with the empty vector. This result obtained using anti-CB2 antibody ruled out the possibility that the interaction of Hsp90 with CB2 might be caused by the presence of FLAG epitope, and it indicated that the interaction between Hsp90 and CB2 receptor was indeed specific. Second, to further verify the interaction of Hsp90 with CB2 receptor, coimmunoprecipitation and immunoblotting experiments were carried out in a reverse order; i.e., anti-Hsp90 antibody was used for immunoprecipitation to see whether CB2 receptor could be coimmunoprecipitated. As shown in Fig. 2A, bottom, CB2 band was detected in membrane preparations and immunoprecipitates of HEK293 cells stably expressing FLAG-CB2, but not in membranes and immunoprecipitates prepared from HEK293 cells transfected with the empty vector. These reverse coimmunoprecipitation and immunoblotting experiments provided further evidence for the physical association of Hsp90 with CB2 receptor.
Furthermore, differentiated HL-60 cells were used to test whether the interaction between Hsp90 and CB2 receptor exists in native cells that express endogenous CB2 receptor. As shown in Fig. 2B, both CB2 receptor and Hsp90 proteins were detected in membrane preparations of differentiated HL-60 cells. Using the anti-CB2 antibody, Hsp90 was coimmunoprecipitated with the endogenous CB2 receptor. In contrast, the signal was absent when membrane fractions were immunoprecipitated with a nonrelevant antibody (rabbit anti-mouse IgG) (Fig. 2B, top). Moreover, using the anti-Hsp90 antibody, endogenous CB2 receptor was coimmunoprecipitated with Hsp90 from the solubilized membrane preparations. However, the band was not detected when membrane fractions were immunoprecipitated with a nonrelevant antibody (His-Tag monoclonal antibody; Fig. 2B, bottom). These data from differentiated HL-60 cells clearly demonstrated that the specific interaction between Hsp90 and CB2 receptor exists in native cells expressing endogenous CB2 receptor. It needs to be noted that two bands were detected in the Western blot with the CB2 antibody (Fig. 2, A and B, bottom). These probably correspond to glycosylated and nonglycosylated forms of CB2 receptors, because we have demonstrated previously that CB2 receptors exist in glycosylated and nonglycosylated forms (Feng et al., 2002).
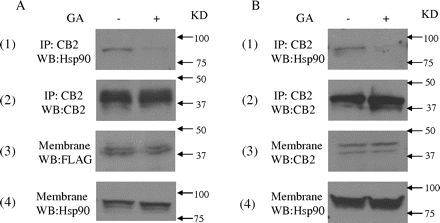
Effect of Geldanamycin on the Interaction between Hsp90 and CB2 Receptor. Geldanamycin, a small molecule that specifically binds to the ATP-binding pocket in Hsp90, but not other chaperone proteins, alters the tertiary structure of Hsp90, and it prevents Hsp90 from interacting with target proteins (Stebbins et al., 1997). In this study, the effect on geldanamycin on the interaction between Hsp90 and CB2 receptor was investigated. In HEK293 cells stably expressing FLAG-CB2, geldanamycin treatment did not significantly affect the amount of CB2 receptor and Hsp90 in membrane fractions (Fig. 3A, 3 and 4). However, treatment with geldanamycin markedly reduced the amount of Hsp90 pulled down by anti-CB2 antibody in coimmunoprecipitation and immunoblotting studies. In addition, similar results were obtained with differentiated HL-60 cells; i.e., geldanamycin treatment significantly reduced the amount of Hsp90 pulled down by anti-CB2 antibody (Fig. 3B). These data further demonstrate that the interaction of Hsp90 with CB2 receptor is specific.
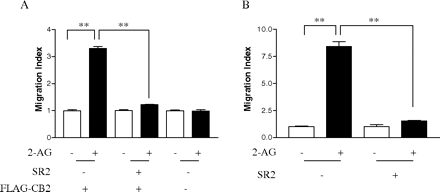
Effect of Geldanamycin on 2-AG-Induced Cell Migration. 2-AG, an endogenous cannabinoid agonist for both CB1 and CB2 receptors, has been shown to cause cell migration in a number of cell types. In this study, we first tested whether 2-AG can induce the migration of HEK293 cells stably expressing FLAG-CB2 and whether the 2-AG-induced cell migration is mediated by CB2 receptors. As shown in Fig. 4A, addition of 2-AG to the bottom chamber of the Transwell cell migration system resulted in a marked enhancement of cell migration from the upper chamber to the underside of the porous membrane. In the presence of 2-AG, the total number of cells migrated (499.17 ± 45.8; mean ± S.E.) was approximately 3 times that of cells migrated in the absence of 2-AG (142.38 ± 14.9; mean ± S.E.). SR144528, a selective CB2 antagonist, was used to examine whether CB2 receptor is involved in 2-AG-induced cell migration. Pretreatment of cells with SR144528 markedly reduced the cell migration induced by 2-AG (Fig. 4A). However, as shown in Fig. 4A, SR144528 alone had no significant effect on the cell migration. Moreover, 2-AG did not induce the migration of HEK293 cells transfected with an empty vector. These data demonstrate that 2-AG-induced cell migration was mediated via CB2 receptors stably expressed in these cells.
Differentiated HL-60 cells are valid models for analyzing human leukocyte migration and chemotaxis (Hauert et al., 2002; Xu et al., 2003). However, the effects of 2-AG on the migration of differentiated neutrophil-like HL-60 cells have not been examined with the Transwell (Boyden chamber) system. To study the downstream signaling pathways of CB2 receptor-mediated migration and the involvement of Hsp90, in this study, we first wanted to test whether 2-AG could induce migration of HL-60 cells differentiated into neutrophil-like cells and whether CB2 receptor is involved. As shown in Fig. 4B, adding 500 nM 2-AG to the bottom chamber of the Transwell cell migration system resulted in a marked enhancement of cell migration from the top chamber to the bottom chamber. In the presence of 2-AG, the total number of cells migrated was approximately 7.5 times that of cells migrated in the absence of 2-AG. In addition, pretreatment of cells with 1 μM SR144528 markedly reduced the 2-AG-induced enhancement of cell migration, whereas SR144528 alone did not affect cell migration (Fig. 4B).
Verification of the interaction between Hsp90 and CB2 receptor. A, confirmation of Hsp90 interaction with CB2 receptor in HEK293 cells stably expressing FLAG-CB2 by coimmunoprecipitation. Top, membranes prepared from HEK293 cells stably expressing FLAG-CB2 and HEK293 cells stably transfected with an empty expression vector were subjected to immunoprecipitation with the anti-CB2 antibody, and they were then immunoblotted with the anti-Hsp90 antibody. Bottom, membranes were subjected to immunoprecipitation with the anti-Hsp90 antibody, and they were then immunoblotted with the anti-CB2 antibody. B, confirmation of Hsp90 interaction with CB2 receptor in differentiated HL-60 cells expressing endogenous CB2 by coimmunoprecipitation. Top, membranes prepared from differentiated HL-60 cells were subjected to immunoprecipitation with the anti-CB2 antibody, and they were immunoblotted with the anti-Hsp90 antibody. Bottom, membranes were subjected to immunoprecipitation with the anti-Hsp90 antibody, and they were then immunoblotted with the anti-CB2 antibody. Control experiments were performed using nonrelevant IgGs (lane 3). The experiment was repeated three times, with similar results.
The effect of geldanamycin on the interaction between Hsp90 and CB2 receptor. A, effect of geldanamycin on the interaction between Hsp90 and CB2 receptor in HEK293 cells stably expressing FLAG-CB2. Cells were treated with 10 μM geldanamycin (GA) for 2 h. Membranes were prepared and subjected to coimmunoprecipitation with anti-CB2 antibody. Then, the samples were immunoblotted with anti-Hsp90 (1) and anti-CB2 antibodies (2). B, effect of geldanamycin on the interaction between Hsp90 and CB2 receptor in differentiated HL-60 cells expressing endogenous CB2. Cells were treated with 10 μM GA for 2 h. Membranes were prepared and subjected to coimmunoprecipitation with the anti-CB2 antibody. Then, the samples were immunoblotted with anti-Hsp90 (1) and anti-CB2 antibodies (2). The experiment was repeated three times, with similar results.
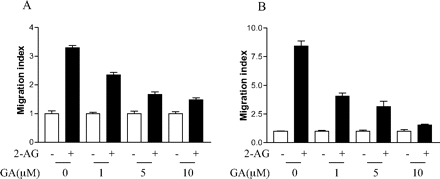
Having shown that geldanamycin can inhibit the interaction of Hsp90 with CB2 receptor, we next examined the possible role of Hsp90 in 2-AG-induced cell migration using geldanamycin. As shown in Fig. 5, A and B, pretreatment of HEK293 cells stably expressing FLAG-CB2 and differentiated HL-60 cells expressing endogenous CB2 with geldanamycin reduced 2-AG-induced cell migration, whereas GA alone had no significant effect on the cell migration. This inhibitory effect of geldanamycin was concentration-dependent in both types of cells. Together, these data on the inhibitory effect of geldanamycin on 2-AG-induced, CB2 receptor-mediated migration of HEK293 and differentiated HL-60 cells suggest that the interaction between Hsp90 and CB2 receptor is crucial for 2-AG-induced cell migration.
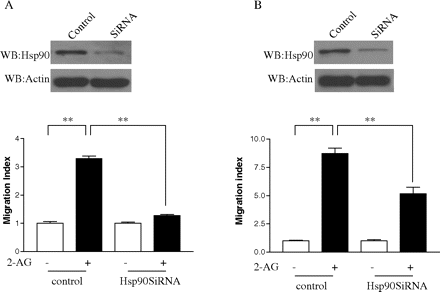
Effect of Hsp90 siRNA on 2-AG-Induced Cell Migration. To examine the effect of decreasing Hsp90 levels on 2-AG-induced cell migration, Hsp90 siRNA was transfected into HEK293 cells stably expressing FLAG-CB2, as well as differentiated HL-60 cells that express endogenous CB2 receptor. In cells that were transfected with Hsp90 siRNA, the expression levels of Hsp90 were significantly decreased (Fig. 6, A and B, top). Likewise, 2-AG-induced cell migration was decreased significantly as a result of Hsp90 knockdown by siRNA (Fig. 6, A and B, bottom). In contrast, Hsp90 siRNA by itself had no significant effect on the cell migration. These data further indicate that Hsp90 plays an important role in 2-AG-induced, CB2 receptor-mediated cell migration.
2-AG-induced, CB2 receptor-mediated cell migration. A, 2-AG-induced migration of HEK293 cells stably expressing FLAG-CB2. 2-AG (500 nM) or vehicle was added to the bottom compartments, and cells were added to the top compartments of the Transwell cell migration system. The system was then incubated for 4 h at 37°C, and the cells migrated to the underside of the porous membrane were determined as described under Materials and Methods. For antagonist treatment, 1 μM SR144528 (SR2) was used. Results are presented as migration index, which is the ratio of cells migrated in the presence of 2-AG versus cells migrated in the presence of vehicle. Data are mean ± S.E. of three independent experiments, each performed in triplicate. **, significant differences (p < 0.01) between experimental groups (ANOVA followed by Newman-Keuls post test). B, 2-AG-induced migration of differentiated HL-60 cells expressing endogenous CB2. 2-AG (500 nM) or vehicle was added to the bottom compartments, and cells were added to the top compartments of the Transwell cell migration system. The system was then incubated for 4 h at 37°C, and the cells migrated to the bottom compartments were determined as described under Materials and Methods. For antagonist treatment, 1 μM SR2 was used. Results are presented as migration index, which is the ratio of cells migrated in the presence of drug versus cells migrated in the presence of vehicle. Data are mean ± S.E. of three independent experiments, each performed in triplicate. **, significant differences (p < 0.01) between experimental groups (ANOVA followed by Newman-Keuls post test).
Effect of geldanamycin on 2-AG-induced cell migration. A, effect of geldanamycin on 2-AG-induced migration of HEK293 cells stably expressing FLAG-CB2. Cells were pretreated with 10 μM geldanamycin for 2 h before detaching from the plate, and then they were subjected to migration assays using the Transwell cell migration system as described under Materials and Methods. Results are presented as migration index, which is the ratio of cells migrated in the presence of drug versus cells migrated in the presence of vehicle. Data shown are mean ± S.E. of three independent experiments, each performed in triplicates. B, effect of geldanamycin on 2-AG-induced migration of differentiated HL-60 cells expressing endogenous CB2. Cells were pretreated with 10 μM geldanamycin for 2 h, and then they were subjected to migration assays using the Transwell cell migration system as described under Materials and Methods. Data shown are mean ± S.E. of three independent experiments, each performed in triplicate.
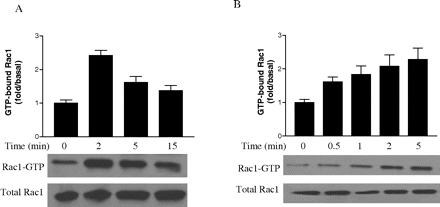
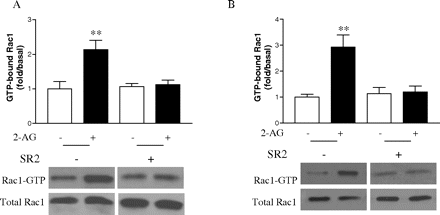
Effect of Geldanamycin on 2-AG-Induced Rac1-GT-Pase Activity. The small GTP-binding protein Rac1 plays a central role in the regulation of the actin-based cytoskeleton and cell movement (Ridley, 2001; Burridge and Wennerberg, 2004; Raftopoulou and Hall, 2004), and Rac1 activation is mediated by Gi protein. Because CB2 receptor is a Gi protein-coupled receptor, we investigated whether Rac1 is involved in the 2-AG-induced cell migration. As shown in Fig. 7A, in HEK293 cells stably expressing FLAG-CB2, by a pull-down assay we observed that stimulation with 2-AG provoked the rapid accumulation of GTP-bound Rac1, which reached a maximum at ∼5 min. Similar results were also obtained in differentiated HL-60 cells expressing endogenous CB2 receptor (Fig. 7B); i.e., treatment with 2-AG led to the increase in Rac1 activity, which reached a plateau at approximately 5 min of 2-AG stimulation. Pretreatment with SR144528 markedly inhibited the activation of Rac1 induced by 2-AG (Fig. 8), indicating that the modulation of Rac1 activity by 2-AG is mediated by CB2 receptor. However, as shown in Fig. 8, SR144528 alone had no significant effect on either GTP-bound Rac1 or total Rac1 levels.
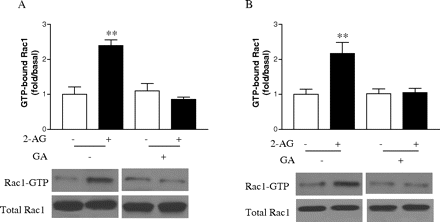
Because we have shown that the interaction of Hsp90 with CB2 plays an important role in 2-AG-induced cell migration, we wondered whether this interaction is also important for 2-AG-induced enhancement of Rac1 activity. This question was answered again by using geldanamycin. As shown in Fig. 9, pretreatment of HEK293 cells stably expressing FLAG-CB2 as well as differentiated HL-60 cells expressing endogenous CB2 with geldanamycin blocked the 2-AG-induced Rac1 activity in both types of cells. However, geldanamycin alone had no significant effect on either GTP-bound Rac1 or total Rac1 levels. These data indicate that by interacting with CB2 receptor, Hsp90 plays an important role in regulating 2-AG-induced increase of Rac1 activity.
Effect of Hsp90 siRNA on 2-AG-induced cell migration. A, effect of Hsp90 siRNA on 2-AG-induced migration of HEK293 cells stably expressing FLAG-CB2. Cells were transfected with 2 μg of Hsp90 siRNA or a scrambled control RNA mixture for 24 h, and then they were subjected to immunoblotting (top) or migration assays (bottom) as described under Materials and Methods. Data for immunoblotting are representative for three independent experiments. Data for migration assays are mean ± S.E. of three independent experiments, each performed in triplicate. **, significant differences (p < 0.01) between experimental groups (ANOVA followed by post test). B, effect of Hsp90 siRNA on 2-AG-induced migration of differentiated HL-60 cells expressing endogenous CB2. Cells were transfected with 2 μg of Hsp90 siRNA or a scrambled control RNA mixture for 24 h, and then they were subjected to immunoblotting (top) or migration assays (bottom) as described under Materials and Methods. Data for immunoblotting are representative for three independent experiments. Migration results are presented as migration index, which is the ratio of cells migrated in the presence of drug versus cells migrated in the presence of vehicle. Data shown for migration assays are mean ± S.E. of three independent experiments, each performed in triplicate. **, significant differences (p < 0.01) between experimental groups (ANOVA followed by Newman-Keuls post test).
Time course of 2-AG-induced Rac1-GTPase activity. Cells were serum-starved for 18 h, and then they were stimulated with 500 nM 2-AG for the indicated times. Rac1-GTP in HEK293 cells stably expressing FLAG-CB2 (A) and differentiated HL-60 cells (B) was assessed by PAK-PBD-GST pull-down assays, respectively. Blots shown are representative of four independent experiments. In the bar graphs, results are normalized to the density of the total Rac1 band in the corresponding samples (n = 4).
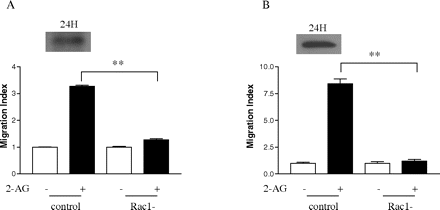
Effect of Dominant-Negative Form of Rac1 on 2-AG-Induced Cell Migration. After finding out that 2-AG stimulates Rac1 activity and the interaction of Hsp90 with CB2 is important for this process, the next question we asked was whether Rac1 is required for 2-AG-induced cell migration. To answer this question, the dominant-negative form of Rac1 was used. Figure 10 demonstrates that 3×HARac1T17N, the dominant-negative form of Rac1, was expressed 24 h after transfection into HEK293 cells stably expressing FLAG-CB2, as well as differentiated HL-60 cells expressing endogenous CB2. It is noteworthy that expression of the dominant-negative form of Rac1 markedly reduced 2-AG-induced cell migration in both types of cells (Fig. 10, A and B). However, expression of the dominant-negative form of Rac1 by itself had no significant effect on the cell migration. These data directly demonstrate that Rac1 plays important roles in 2-AG-induced cell migration.
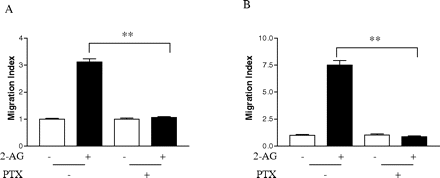
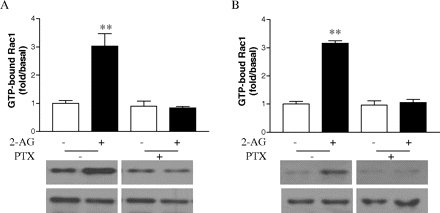
Involvement of Gi in 2-AG-Induced Cell Migration and Rac1-GTPase Activity. Pertussis toxin (PTX), which ADP ribosylates Gαi, thereby uncoupling it from receptor activation (Ui and Katada, 1990), was used to investigate the role of Gi in the process of cell migration stimulated by 2-AG. Treatment with PTX inhibited 2-AG-induced migration of both types of cells (Fig. 11, A and B), whereas PTX alone had no significant effect on the cell migration. These data confirmed that Gi participates in the migratory response initiated by CB2. Because Rac1 plays important roles in 2-AG-induced cell migration, we then investigated whether Gi protein is involved in the 2-AG-induced Rac1 activation. As shown in Fig. 12, pretreatment of two types of cells with PTX attenuated the 2-AG-induced Rac1-GTPase activity. However, PTX alone had no significant effect GTP-bound Rac1 or total Rac1 levels. These data indicate that 2-AG induces Rac1 activation through PTX-sensitive G proteins of the Gi class.
Effect of SR2 on 2-AG-induced Rac1-GTPase activity. Cells were serum-starved for 18 h, and then they were pretreated with 1 μM SR144528 or vehicle at 37°C incubator for 30 min, followed by stimulation with 500 nM 2-AG for 5 min. Next, the Rac1-GTP levels in HEK293 cells stably expressing FLAG-CB2 (A) and differentiated HL-60 cells (B) were measured by PAK-PBD-GST pull-down assay, respectively. Blots shown are representative of four independent experiments. In the bar graphs, results are normalized to the density of the total Rac1 band in the corresponding samples (n = 4).
Effect of geldanamycin on 2-AG-induced Rac1-GTPase activity. Cells were pretreated with vehicle or 10 μM geldanamycin for 2 h, followed by stimulation with 500 nM 2-AG for 5 min, and then the Rac1-GTP levels in HEK293 cells stably expressing FLAG-CB2 (A) and differentiated HL-60 cells (B) were measured by PAK-PBD-GST pull-down assay, respectively. Blots shown are representative of four independent experiments. In the bar graphs, results are normalized to the density of the total Rac1 band in the corresponding samples (n = 4).
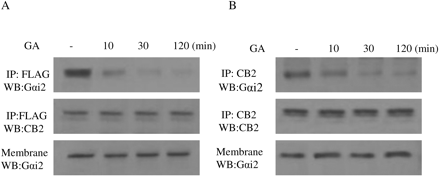
Effect of Geldanamycin on the Interaction between Gαi2 and CB2 Receptor. Because we have shown that the interaction of Hsp90 with CB2 receptor plays an important role in 2-AG-induced cell migration and Rac1 activation, the next question is how Hsp90 modulates 2-AG-induced migration response through interaction with CB2 receptor. It is well known that CB2 receptor coupled to Gi protein, and our data in Figs. 11 and 12 clearly showed that PTX-sensitive G protein of the Gi family is involved in the migratory response mediated by CB2 receptor. A recent article indicated that Hsp90 protein binds to Gαi2 (Falsone et al., 2005). Based on this previous finding and our data in Figs. 11 and 12, we hypothesized that Hsp90 might act as an adaptor between CB2 receptor and Gαi2, thereby regulating Rac1 activation and cell migration. To test this hypothesis, we used geldanamycin to address the role of Hsp90 in the maintenance of CB2/Gαi2 coupling. In HEK293 cells stably expressing FLAG-CB2, geldanamycin pretreatment did not significantly affect the amount of Gαi2 (Fig. 13A). However, treatment with geldanamycin markedly reduced the amount of Gαi2 pulled down by the anti-FLAG antibody in coimmunoprecipitation and immunoblotting studies in a time-dependent manner. In addition, similar results were obtained with differentiated HL-60 cells; i.e., geldanamycin treatment significantly reduced the amount of Gαi2 pulled down by the anti-CB2 antibody (Fig. 13B). These data indicate that Hsp90 might play an important role in keeping the physical coupling between CB2 receptor and its signaling component Gαi2.
Discussion
CB2 is expressed predominantly in cells of the immune system, and it may be involved in a wide range of physiological phenomenon related to immunity (Sugiura et al., 2004; Pertwee, 2005; Buckley et al., 2006; Lunn et al., 2006). Previous studies have shown that endocannabinoid 2-AG induces the migration of several types of immune cells, such as macrophages, monocytes, and eosinophils through a CB2 receptor-dependent mechanism, thereby participating in inflammatory reactions and immune responses (Kishimoto et al., 2003; Oka et al., 2004). In addition, CB2 is aberrantly over expressed in a high percentage of human acute myeloid leukemia cells (Alberich Jordà et al., 2004). Similar to metastasis of other primary tumors, leukemia cells spread throughout the body and invade into secondary tissues. 2-AG has been shown to induce the migration of leukemia cells, and it has been suggested to play an important role in leukemia metastasis (Jordà et al., 2002, 2003; Alberich Jordà et al., 2004).
Effect of the dominant-negative form of Rac1 on 2-AG-induced migration. Cells were transiently transfected with the dominant-negative form of Rac1 plasmid (3×HA Rac1-T17N). At 24 h after transfection, the expression of the dominant-negative form of Rac1, and its effects on 2-AG-induced the migration of HEK293 cells stably expressing FLAG-CB2 (A) and differentiated HL-60 cells (B) were assayed. Data shown for immunoblotting are representative for three independent experiments. Migration results are presented as migration index, which is the ratio of cells migrated in the presence of drug versus cells migrated in the presence of vehicle. Data for migration assays are mean ± S.E. of three independent experiments, each performed in triplicate. **, significant differences (p < 0.01) between experimental groups (ANOVA followed by Newman-Keuls post test).
Effects of PTX on 2-AG induced cell migration. HEK293 cells stably expressing FLAG-CB2 (A) or differentiated HL-60 cells expressing endogenous CB2 (B) were incubated with 100 ng/ml PTX for 18 h, and then they were subjected to migration assays using the Transwell cell migration system as described under Materials and Methods. Migration results are presented as migration index, which is the ratio of cells migrated in the presence of drug versus cells migrated in the presence of vehicle. Data shown are mean ± S.E. of three independent experiments, each performed in triplicate. **, significant differences (p < 0.01) between experimental groups (ANOVA followed by Newman-Keuls post test).
Effects of PTX on 2-AG induced Rac1 GTPase activity. Cells were cultured overnight in serum-free growth medium with 100 ng/ml PTX and stimulated with 500 nM 2-AG for 5 min. The Rac1-GTP levels from HEK293 cells stably expressing FLAG-CB2 (A) and differentiated HL-60 cells expressing endogenous CB2 (B) were measured by PAK-PBD-GST pull down assays. Blots shown are representative of four independent experiments. In the bar graphs, results are normalized to the density of the total Rac1 band in the corresponding samples (n = 4).
Many cellular events depend on the regulated protein-protein interactions. Discovering novel protein interactions should facilitate the understanding of the molecular mechanisms underlying biological processes. In the current study, we first identified Hsp90 as a possible constitutive interacting protein of CB2 receptor by immunoprecipitation and mass spectrometry. The interaction between Hsp90 and CB2 receptor in FLAG-CB2-transfected HEK293 cells was found to be specific, because Hsp90 was not immunoprecipitated with either anti-FLAG or anti-CB2 antibodies in HEK293 cells transfected with an empty vector. The interaction was also seen in differentiated HL-60 cells, which express endogenous CB2 receptors. The rationales for us to choose HL-60 cells were as follows: 1) HL-60 is a human promyelocytic leukemia cell line, from which CB2 receptor was originally cloned (Munro et al., 1993); and 2) differentiated HL-60 cells are established models widely used for studying the mechanisms of leukocyte migration (Hauert et al., 2002; Xu et al., 2003).
Time course of the effect of geldanamycin on the coupling of CB2 receptor with Gαi2. A, HEK293 cells stably expressing FLAG-CB2. B, differentiated HL-60 cells expressing endogenous CB2. Cells were pretreated with 10 μM GA for indicated time and stimulated with 500 nM 2-AG for 5 min. Membranes were prepared and subjected to coimmunoprecipitation with the anti-CB2 antibody. The samples were immunoblotted with the anti-Gαi2 and the anti-CB2 antibodies. The experiment was repeated twice, with similar results.
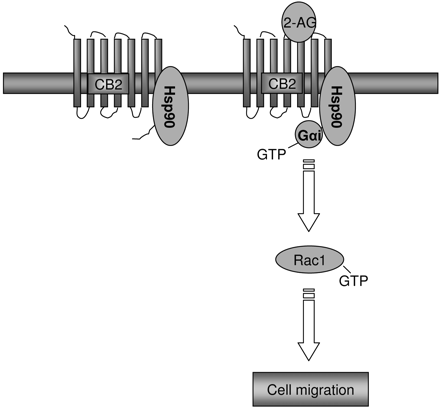
Working model concerning the involvement of Hsp90 in CB2 receptor-mediated cell migration. Hsp90 may function as a scaffold orienting Gαi2 to interact with the CB2 receptor or stabilizing CB2 receptor on the cell surface, thus facilitating CB2-mediated signaling to cell migration through the Gi-Rac1 pathway.
Geldanamycin, a small molecule that specifically binds the ATP-binding pocket in Hsp90 (but not other chaperones), alters the tertiary structure of Hsp90, thus preventing Hsp90 from interacting with target proteins(Stebbins et al., 1997). In this study, pretreatment of cells with geldanamycin inhibited the interaction of Hsp90 with CB2. These results indicate that the interaction between Hsp90 and CB2 is specific and exists at physiological conditions.
In this study, we have confirmed that 2-AG can induce migration of HEK293 cells stably expressing CB2 receptor, as well as HL-60 cells differentiated into neutrophil-like cells. Furthermore, we have demonstrated that CB2 antagonist SR144528 can block 2-AG-induced migration of both types of cells. More importantly, we have shown that pretreatment with geldanamycin markedly reduced the 2-AG-induced, CB2 receptor-mediated migration of HEK293 as well as differentiated HL-60 cells. Furthermore, in Hsp90 knock-down experiments performed with Hsp90 siRNA, we have shown that decreasing the cellular expression of Hsp90 reduced 2-AG-induced migration. These data clearly demonstrate that the interaction between Hsp90 and CB2 is essential for 2-AG-induced, CB2 receptor-mediated cell migration.
How can Hsp90 play a role in the 2-AG-induced, CB2 receptor-mediated cell migration? Before answering this question, we must first understand the signaling pathways of 2-AG-induced cell migration. Rho-families of small GTPases are well known, key regulators in cell migration (Ridley, 2001; Burridge and Wennerberg, 2004; Raftopoulou and Hall, 2004). Previous reports have found that the activation of different component of Rho-families depends upon different signaling pathways; i.e., the activation of RhoA involves the G12/13-mediated pathway, whereas the activation of Rac depends on the Gi-mediated pathway (Xu et al., 2003). It is well known that CB2 receptor is a Gi protein-coupled receptor. As a first step in elucidating the molecular mechanism by which 2-AG induces cell migration, we studied the effects of 2-AG on Rac1 activity. Our data demonstrate that 2-AG induced a time-dependent enhancement of Rac1 activity, and this enhancement was inhibited by CB2 antagonist SR144528, suggesting that Rac1-GTPases may be involved in 2-AG-induced cell migration. Our hypothesis that Rac1-GTPases may be involved in 2-AG-induced cell migration is further supported by our data demonstrating that the dominant-negative form of Rac1 markedly inhibited 2-AG-induced cell migration. To our knowledge, this study is the first to directly establish the involvement of Rac1 in cannabinoid-induced cell migration using the dominant-negative form of the Rho-families of small GTPases. These data are consistent with a recent report on the effect of the dominant-negative form of Rac1 on N-formyl-l-methionyl-l-leucyl-l-phenylalanine-induced cell migration (Pestonjamasp et al., 2006). In the current study, we have found that pretreatment with PTX inhibited the cell migration induced by 2-AG in both types of cells. These data confirm that Gi participates in the migratory response mediated by CB2 receptor. Furthermore, pretreatment of both two types of cells with PTX attenuated the 2-AG-induced Rac1 GTPase activity, again confirming the involvement of Gi protein. Taken together, our experimental data support the hypothesis that 2-AG-induced, CB2 receptor-mediated cell migration involves Rac1 activation through PTX-sensitive G proteins of the Gi class.
In this study, we have found that pretreatment of cells with geldanamycin inhibited 2-AG-induced activation of Rac1. In addition, we have shown that pretreatment of cells with geldanamycin decreased 2-AG-induced cell migration. A previous study has reported the effects of geldanamycin on proteinase-activated receptor-1-stimulated, thrombin receptor-mediated changes in cell morphology and cytoskeleton (Pai et al., 2001). To our knowledge, the current study is the first to demonstrate a modulatory effect of geldanamycin on ligand-induced, GPCR-mediated cell migration. These data on the modulatory effects of geldanamycin on 2-AG-induced Rac1 activity, and 2-AG-induced cell migration, support the hypothesis that Hsp90 regulates 2-AG-induced cell migration by modulating 2-AG-induced Rac1 activity.
How might Hsp90 modulate 2-AG-induced Rac1 activity? The current study indicated that Hsp90 acts upstream of Rac1, because geldanamycin blocked 2-AG-induced activation of Rac1. In a previous publication on Hsp90 interactome analysis, it has been shown that Hsp90 can interact with Gαi2 (Falsone et al., 2005). Using PTX, in this study we also confirmed the involvement of Gi protein in 2-AG-induced, CB2 receptor-mediated Rac1 activation and cell migration. Therefore, we hypothesized that Hsp90 may regulate CB2-mediated Rac1 activation through modulation of the coupling between CB2 receptor and Gαi2. This hypothesis is supported directly by our experimental data demonstrating that treatment with geldanamycin decreased the physical coupling of CB2 receptor with Gαi2. Therefore, it is likely that Hsp90 serves as a scaffold to keep the CB2 receptor and its signaling components, including Gαi2, in proximity, thus ensuring proper signaling transduction. To our knowledge, this is the first time that Hsp90 has been shown to play a critical role in the coupling of a GPCR to its G protein. However, it is also possible that Hsp90 may be necessary to stabilize the receptor on the cell surface.
Compared with other heat shock proteins, Hsp90 is distinguished by its specific interactions with various signaling proteins and its important role in signal transduction under nonstress conditions. Our studies add support to this premise by demonstrating that geldanamycin not only inhibited the interaction between CB2 receptor and Hsp90 but also prevented the CB2/Gαi2 coupling, the activation of Rac1, and the subsequent cell migration induced by 2-AG.
In conclusion, as outlined in Fig. 14, this study demonstrates that Hsp90 is essential for CB2-mediated cell migration, and it reveals a novel role of Hsp90 in the signaling events mediated by a G protein-coupled receptor.
Footnotes
-
This work is supported in part by National Institutes of Health grants DA11551 and EY13632.
-
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
-
doi:10.1124/mol.107.036566.
-
ABBREVIATIONS: GPCR, G protein-coupled receptor; CB, cannabinoid; 2-AG, 2-arachidonoylglycerol; Hsp90, 90-kDa heat shock protein; HEK, human embryonic kidney; SR144528, SR2, N-[(1S)-endo-1,3,3-trimethyl-bicyclo-[2.2.1]-heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide; siRNA, short interfering RNA; DMEM, Dulbecco's modified Eagle's medium; DMSO, dimethyl sulfoxide; MALDI-TOF, matrix-assisted laser desorption ionization/time of flight; MS, mass spectrometry; PAGE, polyacrylamide gel electrophoresis; ACN, acetonitrile; TBS-T, Tris-buffered saline/Tween 20; ANOVA, analysis of variance; PTX, pertussis toxin; GA, geldanamycin; PAK-PBD-GST, p21-activated kinase-PAK binding domain-glutathionetransferase.
- Received March 28, 2007.
- Accepted August 14, 2007.
- The American Society for Pharmacology and Experimental Therapeutics