Visual Overview
Abstract
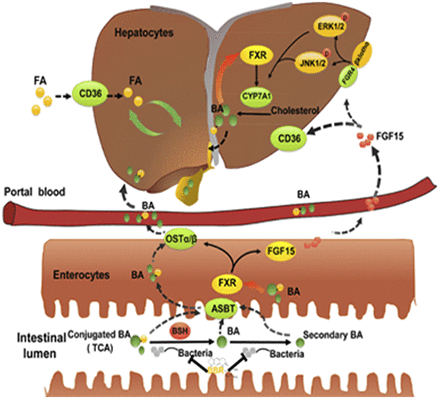
Previous studies suggest that the lipid-lowering effect of berberine (BBR) involves actions on the low-density lipoprotein receptor and the AMP-activated protein kinase signaling pathways. However, the implication of these mechanisms is unclear because of the low bioavailability of BBR. Because the main action site of BBR is the gut and intestinal farnesoid X receptor (FXR) plays a pivotal role in the regulation of lipid metabolism, we hypothesized that the effects of BBR on intestinal FXR signaling pathway might account for its pharmacological effectiveness. Using wild type (WT) and intestine-specific FXR knockout (FXRint−/−) mice, we found that BBR prevented the development of high-fat-diet–induced obesity and ameliorated triglyceride accumulation in livers of WT, but not FXRint−/− mice. BBR increased conjugated bile acids in serum and their excretion in feces. Furthermore, BBR inhibited bile salt hydrolase (BSH) activity in gut microbiota, and significantly increased the levels of tauro-conjugated bile acids, especially tauro-cholic acid(TCA), in the intestine. Both BBR and TCA treatment activated the intestinal FXR pathway and reduced the expression of fatty-acid translocase Cd36 in the liver. These results indicate that BBR may exert its lipid-lowering effect primarily in the gut by modulating the turnover of bile acids and subsequently the ileal FXR signaling pathway. In summary, we provide the first evidence to suggest a new mechanism of BBR action in the intestine that involves, sequentially, inhibiting BSH, elevating TCA, and activating FXR, which lead to the suppression of hepatic expression of Cd36 that results in reduced uptake of long-chain fatty acids in the liver.
Footnotes
- Received September 2, 2016.
- Accepted December 5, 2016.
↵1 R.S., N.Y., and B.K. contributed equally to this work.
This study was financially supported by the National Natural Science Foundation of the People’s Republic of China [81573495, 81530098], the Key Technology Projects of China “Creation of New Drugs” [2015ZX09501001], the Project for Jiangsu Province Key Laboratory of Drug Metabolism and Pharmacokinetics [BM2012012], and in part by the National Institutes of Health [R01GM104037]. The authors received financial support from the China Scholarship Council [201407060027].
- Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics
MolPharm articles become freely available 12 months after publication, and remain freely available for 5 years.Non-open access articles that fall outside this five year window are available only to institutional subscribers and current ASPET members, or through the article purchase feature at the bottom of the page.
|