Visual Overview
Abstract
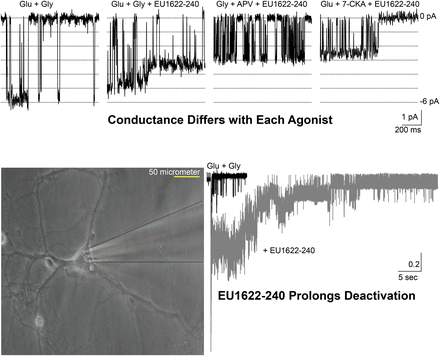
N-methyl-D-aspartate receptors (NMDARs) are ionotropic glutamate receptors that mediate a slow, Ca2+-permeable component of excitatory neurotransmission. Modulation of NMDAR function has the potential for disease modification as NMDAR dysfunction has been implicated in neurodevelopment, neuropsychiatric, neurologic, and neurodegenerative disorders. We recently described the thieno[2,3-day]pyrimidin-4-one (EU1622) class of positive allosteric modulators, including several potent and efficacious analogs. Here we have used electrophysiological recordings from Xenopus oocytes, human embryonic kidney cells, and cultured cerebellar and cortical neurons to determine the mechanisms of action of a representative member of this class of modulator. EU1622-240 enhances current response to saturating agonist (doubling response amplitude at 0.2–0.5 μM), slows the deactivation time course following rapid removal of glutamate, increases open probability, enhances coagonist potency, and reduces single-channel conductance. We also show that EU1622-240 facilitates NMDAR activation when only glutamate or glycine is bound. EU1622-240-bound NMDARs channels activated by a single agonist (glutamate or glycine) open to a unique conductance level with different pore properties and Mg2+ sensitivity, in contrast to channels arising from activation of NMDARs with both coagonists bound. These data demonstrate that previously hypothesized distinct gating steps can be controlled by glutamate and glycine binding and shows that the 1622-series modulators enable glutamate- or glycine-bound NMDARs to generate open conformations with different pore properties. The properties of this class of allosteric modulators present intriguing therapeutic opportunities for the modulation of circuit function.
SIGNIFICANCE STATEMENT NMDA receptors are expressed throughout the central nervous system and are permeable to calcium. EU1622-240 increases open probability and agonist potency while reducing single-channel conductance and prolonging the deactivation time course. EU1622-240 allows NMDA receptor activation by the binding of one coagonist (glycine or glutamate), which produces channels with distinct properties. Evaluation of this modulator provides insight into gating mechanisms and may lead to the development of new therapeutic strategies.
Footnotes
- Received August 23, 2024.
- Accepted October 9, 2024.
This work was supported by National Institutes of Health National Institute on Neurologic Disorders and Stroke [NS111619] (to S.F.T.).
S.F.T., D.C.L., S.P., R.G.F., and N.S.A. are coinventors on Emory-owned intellectual property that includes EU1622-240. S.F.T. is a member of the SAB for Sage Therapeutics, Eumentis Therapeutics, Neurocrine Biosciences, the GRIN2B Foundation, the CureGRIN Foundation, and CombinedBrain. S.F.T. is a consultant for GRIN Therapeutics, a cofounder of NeurOp, Inc. and Agrithera, and a member of the board of directors for NeurOp Inc. S.F.T. is the principal investigator on a research grant from GRIN Therapeutics to Emory. D.C.L. is on the board of directors for NeurOp Inc.
↵
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
- Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics
MolPharm articles become freely available 12 months after publication, and remain freely available for 5 years.Non-open access articles that fall outside this five year window are available only to institutional subscribers and current ASPET members, or through the article purchase feature at the bottom of the page.
|