Abstract
Melatonin N-acetyl-5-methoxytriptamine is an ancient molecule which synchronizes the internal biologic activity with the environmental photoperiod. It is synthesized by the pineal gland during the night and released to the general circulation, where it reaches nanomolar concentrations. The indolamine acts through melatonin receptors and binds to different proteins such as calmodulin: a phylogenetically conserved protein which is the main transductor of the calcium signaling. In this review, we will describe evidence supporting that melatonin binds to calmodulin in presence of calcium, and we discuss the effects of this indolamine on the activity of calmodulin kinase II as an inhibitor and as stimulator of calmodulin-dependent protein kinase II activity. We also provide a literature review supporting the relevance of melatonin binding to calmodulin in the regulation of circadian rhythms in unicellular organisms, as well as in neuronal development in mammals as an ancient, conserved mechanism. Finally, we highlight the importance of antioxidant effects of melatonin on calmodulin preservation.
SIGNIFICANCE STATEMENT This review compiled evidence supporting that melatonin binds to calmodulin. We discuss the dual effect of melatonin on the activity of calmodulin kinase II, the possible mechanisms involved, and the relevance on regulation of circadian rhythms and neurodevelopment. Finally, we describe evidence supporting that the binding of melatonin to calmodulin hydrophobic pockets may prevent the oxidation of methionine species with a shielding effect that preserves the functionality of calmodulin.
Melatonin
Structural Features and Physiologic Effects
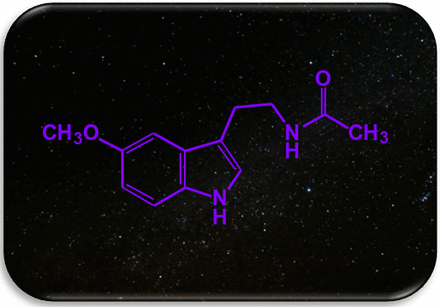
Melatonin, N-acetyl-5-methoxytriptamine (MEL) (Fig. 1) is a derivative of tryptophan. MEL is synthesized in the pineal gland during the night and released to the general circulation and to the cerebrospinal fluid where it reaches picomolar and nanomolar concentrations, respectively (Tan et al., 2023). The enzymes responsible for its biosynthesis are arylkylamine N-acetyl transferase, serotonin N-acetyltransferase, hydroxy indole-O-methyl transferase, or acetyl serotonin N-methyltransferase (Singh and Jadhav, 2014). MEL is metabolized mainly in the liver and is excreted in the urine as 6-hydroxy-melatonin, N(1)-acetyl-N(2)-formyl-5-methoxykynuramine and N(1)-acetyl-5-methoxykynuramine (Silva et al., 2004). This hormone acts mainly by binding to membranal receptors. However, evidence indicates that MEL binds to intracellular proteins among which we find calmodulin (for a review see Liu, et al., 2019).
Melatonin structure, N-acetyl-5-methoxytriptamine is synthesized during the night in the pineal gland. Melatonin synthesis is suppressed by the light perceived by the retina and this signal is transduced to the pineal gland by a noradrenergic pathway.
At the subcellular level, MEL modulates the structural organization of the cell. The indolamine induces cytoskeletal rearrangements influencing microfilament, microtubule, and intermediate filament organization (Benítez-King, 2006). This complex structure plays a key role in multiple functions, such as proliferation, organelle transport, neuronal connectivity, and neurodevelopment (Miranda-Riestra et al., 2022).
MEL has, according to various descriptions, a broad range of physiologic effects. Among its myriad functions are the synchronization of circadian and seasonal rhythms, such as the sleep-wake cycle rhythm, body core temperature, and reproduction (Hardeland et al., 2003). It also modulates the immune responses, and acts as a neurotrophic factor, among other actions (Hardeland, et al., 2011; Miranda-Riestra et al., 2022). In addition, the utility of MEL has been extensively described, for instance in in pharmacological doses for the treatment of jet lag, as a sleep promotor, for the treatment of depression and stress, as well as an antioxidant (Reiter et al., 1995; Herxheimer and Petrie, 2002; Catena-Dell’Osso et al., 2012; Zhang and Zhang, 2014; Maruani et al., 2023).
Mechanisms of Action of Melatonin and Protein Interactions
Predominant physiologic effects of MEL, such as circadian rhythm synchronization and sleep, are produced by stimulation of MEL receptors MT1, MT2, and G protein-coupled receptor 50, which belong to the G protein-coupled receptor superfamily. These receptors interact with one another to form homo- and heterodimers, which are components of larger molecular complexes of proteins (Gerbier et al., 2021). MEL signaling pathways are intricate, and among the best characterized are the cAMP and the inositol triphosphate/dopamine antagonist 6signaling pathways (Luchetti et al., 2010).
Additionally, MEL serves as an antioxidant maintaining cellular redox balance by inducing the expression of enzymes, such as catalase and superoxide dismutase (Tomás-Zapico and Coto-Montes, 2005).
Currently, it is known that MEL interacts with different proteins in a broad range of affinities, from nanomolar to millimolar concentrations. The functional relevance of MEL interaction at micro and millimolar concentrations is a topic still under discussion. Some evidence suggests that melatonin is synthesized in extrapineal sites. However, its concentration is unknown. Reports of melatonin quantitation in brain tissue indicates 2000 to 5000 pg of MEL per mg of protein (For a review, see Acuña Castroviejo et al., 2014). Other studies have described nanodomains in which MEL can be accumulated in a millimolar magnitude order together with its protein effector targets (Loh and Reiter, 2023). However, this interpretation is speculative and more studies are necessary to clarify whether MEL is accumulated inside of the lipid nanodomains. However, Gonyaulax polyedra produces millimolar concentrations of melatonin in response to drastic changes in the environmental temperature. The attributed function of MEL at this concentration is that it acts as an antioxidant and serves for the algae adaptation to adverse environmental conditions (Antolín et al., 1997).
Among these are membrane MEL receptors MT1 and MT2 (MEL binds with, nanomolar affinity) and the enzyme quinone reductase 2, originally identified as the third MEL receptor (MT3) (MEL binds with micromolar affinity) (Dubocovich, 1988; Nosjean et al., 2000). Moreover, MEL binds to other enzymes such as metaloproteinase-9, phosphoprotein phosphatase 2A, pepsin, and transporters, such as the glucose transporters (GLTs) and the oligopeptide transporters 1/2 (MEL binds with millimolar affinity) (Huo et al., 2017).
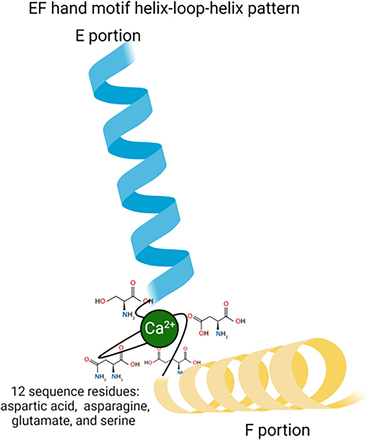
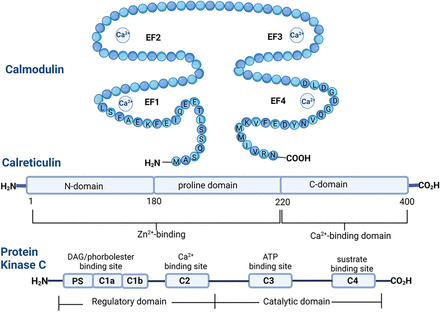
Interestingly, MEL binds to the calcium acceptor proteins: calmodulin (CaM), protein kinase C (PKC) and calreticulin (For a review, see Liu et al., 2019). These three proteins participate in calcium signal transduction as well as regulating calcium concentrations within the cell (Elíes et al., 2020). Notably, these proteins have different structural features, yet they all share an alpha helix structure. CaM contains four EF hand structures formed by two alpha helixes where calcium binds (Fig. 2).
Schematic representation of the EF hand structure of calmodulin formed by two alpha helix loops. The calcium binding site is shown marked in green.
PKC features a C2 domain and an alpha helix structure near its C-terminal domain. In contrast, calreticulin includes a proline-rich β-hairpin arm and a C-terminal tail composed of a β-sheet sandwich and an α-helix (Fig. 3) (Elíes et al., 2020).
Schematic draw of the structure of three calcium and melatonin binding proteins. Calmodulin has two EF hand structures formed by two alpha helixes placed near -NH2 terminal and -CO2H terminal respectively, and four calcium binding sites. Calreticulin has a proline-rich β-hairpin arm and a C-terminal tail composed of a β-sheet sandwich and an α-helix. The alpha isoform of PKC contains a C2 domain and an alpha helix structure near C-terminal domain. Modified from (Elíes et al., 2020).
The protein that has been seldomly studied regarding its interaction with MEL is PKC. Nevertheless, it has been established that MEL enhances the binding of phorbol ester phorbol myristate acetate to PKC and stimulates its activity in vitro, as phorbol myristate acetate does (Antón-Tay et al., 1998). These findings suggest that MEL likely binds to the DAG (diacylglycerol) binding site in PKC alpha, as it selectively activates the alpha isoform of PKC and has no effect on the epsilon isoform, which does not bind calcium (Benítez-King et al., 2001). Altogether, the data suggest that MEL can interact with Ca2+-binding proteins through a hydrophobic motif. In fact, the calcium binding capability of these proteins suggests that MEL may modulate calcium signaling and its intracellular concentrations. Although MEL binds to several proteins, in this review we will focus on the MEL interaction with CaM and its possible physiologic implications.
Calmodulin an Ancient Molecule
Evidence seems to indicate that natural selection allowed the conformational structure and the amino acid sequence of CaM to be conserved from unicellular organisms to invertebrates and vertebrates, evincing that CaM evolved for more than one billion years. This protein expresses a slow rate of replacement in its amino acid composition (Baba et al., 1984). Therefore, CaM was phylogenetically conserved and has a molecular weight of 17 000 kDa and an isoelectric point of 3.5. In mammals, three similar CaM genes have been found in humans and rodents. This supports the idea that these genes are product of evolutionary specialization, and their proteins participate in functions ranging from chain oxidoreduction reactions in unicellular organisms to a more complex biologic process such as synaptic plasticity in mammals (Friedberg and Rhoads, 2001).
CaM is the main transductor of calcium signaling and senses oscillations of intracellular concentrations of this important cation (Elíes et al., 2020). This protein modulates the action of different enzymes; it is also a regulator of calcium transport, and it is found within the cells in a range of nanomolar concentrations. Furthermore, Ca2+-CaM plays a crucial role in the hippocampus, participating in learning and memory, which are adaptative physiologic processes that allow the survival of species (Kool et al., 2019; Yasuda et al., 2022). Amino acid substitutions occur in fungi, plants, and invertebrates, and the amino acid sequence of CaM in vertebrates becomes completely invariant. We suggest that these features have been the result of evolutionary pressures that give CAM various functions throughout evolution (Friedberg and Rhoads, 2001).
The spatial structural conformation of CaM has also been conserved throughout evolution. CaM contains two globular lobes separated by a flexible alpha helix structure of 20 amino acids (Villalobo et al., 2019). CaM binds two atoms of Ca2+ with high affinity in each EF-hand motif, found in the -CO2H terminal domain, and two others with lower affinity in the EF-hand structures, formed in the -NH2 terminal domain (Lakowski et al., 2007). CaM adopts a double dumbbell shape after calcium binding (Holo-CaM), whereas in the absence of Ca2+, CaM (EGTA-CaM or Apo-CaM) has a random structure (Vigil et al., 2001; Villalobo et al., 2019). Holo-CaM and Apo-CaM have different functions within the cell. CaM activated by calcium inhibits tubulin polymerization and participates in muscle contraction, as well as in glycogen metabolism. While Apo-CaM free of calcium, participates in NO synthesis, cAMP production, and releasing calcium from the sarcoplasmic reticulum (Jurado et al., 1999; Villalobo et al., 2019).
In multicellular complex organisms, CaM can bind to different structural proteins and enzymes (approximately 300) (Klee and Means, 2002) located in various cellular compartments, including cytosol, plasma membrane, cytoskeletal structure, and organelles such as mitochondria, Golgi, and the endoplasmic reticulum. Examples of CaM-binding proteins are the calmodulin kinase II (CaMKII), alpha chain of actin, CaM-kinase IV, and calcineurin (O’Connell et al., 2010).
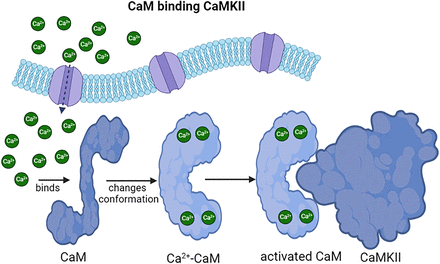
Importantly, CaM adopts diverse structural conformations upon interacting with the CaM binding proteins and CaM antagonists. Currently, approximately 50 different structural conformations of CaM have been described binding to its target proteins (Park et al., 2008; Best and Hummer, 2009; Shukla et al., 2016). The multiple conformations adopted by CaM provide great versatility in transducing calcium signaling into various subcellular compartments (Chen et al., 2006), Fig. 4. In addition, Ca2+-CaM undergoes other conformational changes upon binding to its target proteins (Ikura and Ames, 2006).
Calmodulin binds to its target enzymes and stimulates their activity. Calcium concentrations increase within the cell by calcium entrance through channels or by release from the endoplasmic reticulum after activation of specific signaling pathways. When calcium binds to CaM, it adopts a double-shaped bell. Activated CaM can bind to its target enzymes, such as CaMKII to stimulate its activity.
Calmodulin Participation in Ancient Biologic Rhythms
As we mentioned CaM is a ubiquitous protein conserved throughout evolution. Its amino acid sequence is similar in eukaryotes and just like MEL, CaM is also present (Hoeflich and Ikura, 2002) in unicellular organisms, plants, vertebrates, and invertebrates (Lonergan, 1990; ÓNeill and DeGrado, 1990). Evidence suggests that this protein played a role in regulating the circadian rhythm of photoautotroph unicellular algae, such as Euglena gracilis, in ancient times. The algae had changes in the morphologic and photosynthetic capacity according to the phase of the light/dark cycle. Photosynthesis implicates oxygen production, an essential function for cell survival and takes place in the middle of the light phase. At the same time, the algae adopt an enlarged shape. In contrast, during the dark phase (which marks the final phase of photosynthesis, in which light-independent reactions occur), photosynthetic capability decreases, and the algae changes to a spherical shape (Lonergan, 1983, 1985, 1986). Cell shape changes and photosynthesis is produced in the presence of calcium (Lonergan, 1983). Alterations of both processes occur when intracellular calcium concentrations decrease in the presence of the calcium chelator EGTA, or with the disruption of the signaling pathway of inositol triphosphate calcium signaling (Lonergan, 1990). Importantly, the incubation of the Euglena gracilis with Ca2+ channel blockers, such as verapamil, nifedipine, or the intracellular Ca2+ antagonist TMB-8, caused cell rounding during the middle of the light phase, similar to the rounding occurring during the dark phase of the photoperiod (Lonergan and Williamson, 1988). Similarly, the CaM antagonists, trifluoperazine and chlorpromazine caused the algae to adopt a spherical shape, similar to the one in the dark phase (Lonergan, 1985). Thus, CaM antagonists disrupted the rhythmicity of the algaés photosynthetic cycle (Lonergan, 1986). In short, these results suggested that the antagonist activity of CaM participates in the synchronization of the photosynthetic reactions of the biologic clock.
There is no evidence of MEL secretion in Euglena gracilis. However, it has been described that this indolamine is synthesized in the unicellular algae Gonyaulax polyedra during the dark phase and under adverse meteorological conditions, such as low temperatures. MEL in these conditions elicits the algae encystment allowing its survival. Interruption of the dark phase with light for 2 hours blocked the encystment (Balzer and Hardeland, 1991, 1992; Hardeland et al., 1995). Thus, MEL serves as a mediator during the dark phase in unicellular organisms, as it does in higher organisms like vertebrates. Remarkably, CaM plays a key role in this important process that allows the survival of unicellular organisms to adverse environmental conditions, such as encystment (Matsuoka, 2021).
Biologic rhythms are known to be fundamental for mental and physical health in superior organisms, such as mammals. Experiments with CaM antagonist such as trifluoperazine and W-7 suggested the participation of CaM in the regulation of circadian rhythms (Shibata and Moore, 1994). CaM antagonists produce phase shifting effects on locomotor activity of rats and in hypothalamic slices, which include the suprachiasmatic nucleus (Shibata and Moore, 1994). Moreover, evidence indicates that CaM participates in circadian rhythms through activation of CaM kinases, increasing phosphorylation of cAMP response element-binding protein, which is a mediator in the photic entrainment of the circadian clock (Golombek and Ralph, 1995). Synchronization of the biologic systems with the environment has been conserved in non-human primate species (Muñoz-Delgado et al., 2005) and in other species, such as wolves (Sánchez-Ferrer et al., 2016). Physical and mental illness in humans are associated with out-of-synchrony (Apiquian et al., 2008; Rothschild-Fuentes et al., 2013) and this can also be observed because of niche changes in primates (Muñoz-Delgado and Corsi-Cabrera, 2010).
All this evidence allows us to suggest that the interaction of CaM with MEL might be an ancient mechanism that synchronizes the unicellular organisms with the photoperiod for a better environmental adaptation and organism survival. Furthermore, this interaction has been conserved in mammals to modulate cognition for survival improvement (Balzer and Hardeland, 1991, 1992; Golombeck and Ralph, 1995, 1994; Hardeland et al., 1995; Yasuda et al., 2022).
Melatonin Binding to Calmodulin
Hydrophobic molecules of low molecular weight bind to CaM and inhibit its activity. These molecules are known as CaM antagonists and prevent the interaction of CaM with its target enzymes. Interaction of CaM with CaM antagonists causes modifications in the structural conformation of this protein (Vertessy et al., 1998: Harmat et al., 2000). These molecules have different chemical structures; examples of such molecules are the phenothiazine derivative trifluoperazine, chlorpromazine, N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W7), and vinblastine. Similarly, small peptides, such as melittin and the calmodulin-binding peptide derived from the C-terminus of skeletal muscle myosin light chain kinase, bind to CaM (Dürvanger and Harmat, 2019; Dürvanger et al., 2023).
X-Ray crystallography and circular dichroism in solution allowed the identification of the binding sites of CaM antagonists (Craven et al., 1996; Vertessy et al., 1998). In this context, trifluoperazine binds to the two hydrophobic binding sites located at each of the globular domains of CaM placed near the amine and carboxylic terminal sites (Vertessy et al., 1998).
The MEL interaction with CaM had been studied with different approaches. Methods like the measurements of the relative mobility of proteins separated by one-dimensional gel electrophoresis, the ligand-binding assay, spectrofluorometric and nuclear magnetic resonance spectroscopy (NMR) techniques, and theoretical molecular dynamics simulations have been used to study MEL binding to CaM.
Initially, the interaction of MEL with CaM was studied by measuring the relative mobility of this protein separated by gel electrophoresis in the presence of calcium. MEL-reduced shift in CaM mobility was observed with MEL 10−5 M, like the shift in the relative mobility of CaM caused by the absence of calcium and with the calcium chelator EGTA (Benítez-King et al., 1991; Viviano et al., 2016).
MEL binding to the CaM incorporated into liposomes was assessed in solution and in vitro by fast ultrafiltration ligand-binding assay. The authors used a purified CaM and radioactive MEL (Benítez-King et al., 1993). The binding of MEL was saturable, reversible, ligand selective, and was displaced by 6-chloromelatonin, 6-hydroxymelatonin, luzindole, and the CaM antagonist trifluoperazine. That work demonstrated a single binding site on CaM, with a kDa of 188 pM and a binding capacity of 35 pmoles/µg (Benítez-King et al., 1993; Romero et al., 1998).
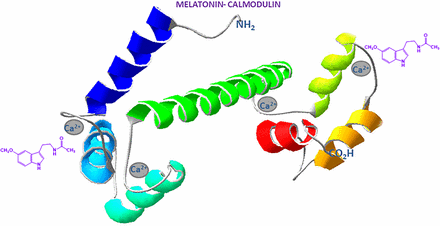
In silico studies have demonstrated that melatonin binds to Ca2+ CaM through hydrophobic interactions involving the indole group, which interacts with the aliphatic and aromatic amino acids situated within the hydrophobic pocket of the dumbbell shaped CaM, activated by calcium (Turjanski, et al., 2007; Meytal and Zisapel, 2007). Similarly, melatonin binds to Ca2+ CaM at the hydrophobic pocket (Fig. 5) in the –CO2H pocket that also binds to MEL helped by a basic group located at the –NH2 terminal domain (Prozialeck and Weiss, 1982).
Melatonin binds to calmodulin in two hydrophobic pockets located near the calcium binding sites placed in the EF hand structures. Gray circles represent calcium binding sites. Green ribbon represents the alpha helix structure placed in the middle of the protein.
Another approach for the study of MEL interaction with CaM was using fluorescence and NMR as well as theoretical molecular dynamics simulations (Turjanski et al., 2004; 2007). These methods demonstrated that MEL binds to CaM and that the binding is weak and calcium dependent. This approach also showed that MEL binds in the two globular sites, formed each by two EF-hand structures located very near the calcium binding sites. Molecular docking shows MEL inside of the hydrophobic pocket with the O-methyl group pointing to the bottom of the pocket and the aliphatic chain located in the entrance (Turjanski et al., 2007). The indolic nitrogen also forms hydrogen bridges with the carbonyl of the alanine 128, like in the binding of the CaM antagonist W7 to CaM (Osawa et al., 1998; Turjanski et al., 2004).
NMR provides helpful information only for relatively strong complexes (Kd < 10 μM). (Ouyang and Vogel, 1998; Turjanski et al., 2004). Another approximation to study MEL binding to CaM was done in silico. The interaction between CaM and MEL is stabilized when CaM is bound to the enzyme CaMKII. The authors observed MEL interactions in the millimolar range, and the Kd values align with the NMR experiments (Meytal and Zisapel, 2007).
Recently, our group found that MEL interacts with CaM in vivo. Using confocal microscopy, we found that in brain hippocampal slices of mice injected with 16 mg/kg melatonin, the indolamine and CaM showed increased colocalization sites. This result indicates that MEL and CaM are in proximity and therefore suggest that their interaction in vivo occurs (Argueta et al., 2022).
Altogether, the results obtained with different experimental models, such as in vitro experiments with purified components, plasma membrane fractions, rodent models treated with MEL, as well as theoretically by computational methods, support that the indolamine interacts and binds to CaM in two different hydrophobic pockets and by intermolecular interactions, such as hydrogen bonds similarly to the binding of CaM antagonists.
Inhibitory Effects of Melatonin on Calmodulin Activity: Physiologic Meaning
Evidence indicates that MEL produces a bimodal effect on CaM activity. In some experimental conditions, MEL antagonize the activity of this protein, while, in others, the indolamine stimulates its activation and consequently increases the activity of the Ca2+-CaM binding enzymes.
In this regard, MEL inhibits in vitro assays, the activity of enzymes activated by Ca2+-CaM. Among these are cAMP-phosphodiesterase, CaM dependent protein kinase II, cerebellar nitric oxidase synthase, estrogen receptor alpha, and acetylcholine receptors in rat myotubes. The inhibition of these enzymes by MEL was dose-dependent and observed in the range of physiologic serum concentration of MEL at night (1–10 nM) (Table 1) (Benítez-King et al., 1991; Benítez-King et al., 1996; Pozo et al., 1994; Del Río et al., 2004; De Almeida et al., 2005). However, calcineurin and CaMKII activity measured with the syntyde-2 substrate are inhibited with concentrations in the millimolar and micromolar range. Thus, it is necessary to make more experiments to explore the physiologic meaning of the inhibitory effects of melatonin to calmodulin at these concentrations.
Target proteins activated by calmodulin and inhibited by melatonin
Inhibition by MEL was dose-dependent and observed in the range of physiologic serum concentration of MEL at night (1–10 nM) (Table 1).
CaM antagonists, like MEL, inhibit the activity of the aforementioned CaM-dependent enzymes. trifluoperazine showed an inhibitory effect on cAMP phosphodiesterase activity with an IC50 value of 10−5 M. CaM-kinase II activity was also inhibited by this phenothiazine, and by the naphthalene derivative W7 and compound 48/80, while the activity of the estrogen receptor alpha and acetylcholine receptors were inhibited by calmidazolium (Benítez-King et al., 1991, 1996; Del Río et al., 2004; De Almeida et al., 2005). Importantly, inhibition of CaM by MEL in skeletal muscle cultured cells results in a reduction of both cyclic GMP (cGMP) and cAMP. Consequently, due to the decrease in cAMP, the membrane expression of nicotinic acetylcholine receptors decreases (De Almeida et al., 2005) (Table 1). These inhibitory effects on the enzymes activated by CaM are specific since they were not observed with MEL precursors of serotonin and N-acetyl serotonin or its catabolite, 6-hydroxy-melatonin (Benítez-King et al., 1991; 1996). Thus, these results support that MEL can act as a CaM antagonist in cultured cells and in cell extracts.
Inhibitory actions of MEL to CaM activity have also been reported in biologic processes in a preparation of cytoskeletons in situ, in vitro polymerization assays of tubulin, as well as in brain slices and cancer cultured cells (Huerto-Delgadillo et al., 1994; Fukunaga et al., 2002; Dai et al., 2002).
It is known that CaM activated by Ca2+ caused microtubule disruption at specific intracellular sites through binding to microtubule-associated proteins and tubulin (Kumagai et al., 1986). Ca2+-CaM can regulate the rearrangements of microtubules by inhibition of tubulin polymerization (Keith et al., 1986). MEL blocks tubulin depolymerization caused by Ca2+-CaM, and, instead, the indolamine causes an increase in tubulin polymerization and microtubule enlargement (Huerto-Delgadillo et al., 1994). In cytoskeletons in situ, obtained from N1E-115 cells, MEL completely abolishes the disruptive effect of Ca2+-CaM on tubulin polymerization. In vitro polymerization assays measured by turbidimetry showed similar results. Considering that cytoskeletons in situ are a preparation devoid of plasma membrane, these results support that a direct interaction between Ca2+-CaM and MEL can occur independently of MEL receptors activation. Similar effects were observed with the Ca2+-CaM antagonists trifluoperazine (10−5 M) and compound 48/80 (Huerto-Delgadillo et al., 1994).
CaM antagonist effects were also demonstrated in brain slices. Long-term potentiation is a process of synaptic plasticity in which there is an increase in synaptic communication between neurons in response to high-frequency electrical stimulation. long-term potentiation participates in memory and cognition mechanisms, and it is known that CaMKII plays a key role. MEL and the CaM antagonist calmidazolium, inhibit long-term potentiation in the rat’s suprachiasmatic nucleus. This effect is mediated through the inhibition of CaMKII autophosphorylation, which is a crucial step to fully activate kinases (Fukunaga et al., 2002).
In addition to the evidence described, MEL inhibits cell growth in mammalian cancer cells MCF-7, as well as in MDCK and N1E-115 neuroblastoma cell lines through CaM antagonism (Benítez-King et al., 1994; Dai et al., 2022).
Melatonin Activates CaM Activity: Physiologic Implications
Despite the fact that MEL acts as a CaM antagonist, there are reports also indicating the contrary effect, that is, the indolamine stimulates the activity of CaM target enzymes such as CaMKII. One example is the effect of dendrite formation in the hilar zone of the rat hippocampus. MEL increases dendrite formation and branching at 10−7 and 10−9 M concentrations. This effect was abolished with 4-[(2S)-2-(N-methylisoquinoline-5-sulfonamido)-3-oxo-3-(4-phenylpiperazin-1-yl)propyl]phenyl isoquinoline-5-sulfonate, which is an inhibitor of the activity of CaMKII (Domínguez-Alonso et al., 2012). Moreover, neurogenesis also is inhibited in olfactory neuronal precursors incubated with MEL by 4-[(2S)-2-(N-methylisoquinoline-5-sulfonamido)-3-oxo-3-(4-phenylpiperazin-1-yl)propyl]phenyl isoquinoline-5-sulfonate (Estrada-Reyes et al., 2022), suggesting that the indolamine also stimulates CaMKII activity. Interestingly, luzindole a non-selective antagonist of MEL receptors, partially inhibited the stimulation of neurogenesis caused by MEL, indicating that CaMKII may also be activated downstream MEL receptors (Estrada-Reyes et al., 2022).
An explanation of the opposite effects of MEL on the activity of CaM target enzymes might be that MEL changes intracellular distribution of CaM, causing an increase in the cytosolic relative amounts of these proteins at specific subcellular compartments. In fact, in MDCK, cells concentrate CaM in spots after 3 to 6 hours of incubation (Antón-Tay, 1998). Similarly, MEL increases the amount of CaM in the cytosolic fraction of MCF-7 cancer cells and in hippocampal slices of rat (Dai et al., 2002; Domínguez-Alonso et al., 2015). Thus, these results suggest that MEL recruits and concentrates CaM at specific compartments, increasing the availability of this protein to activate CaM target enzymes and structural proteins. Recent research has described the enrichment of relative amounts of CaMKII in aqueous microdomains, along with its targets and with proteins that can associate with the enzyme. These interactions form multimolecular complexes that collectively work to enhance memory or cognition processes (Loh and Reiter, 2023; Yasuda et al., 2022). Thus, recruitment of CaM in microdomains and subcellular compartments may partially explain the stimulatory effect of MEL on CaMKII (Yasuda et al., 2022). Recently, our group found that the enzymatic activity of CaMKII in presence of Ca2+-CaM and MEL depends on the lipid or aqueous microenvironment. In lipid media, the activity of CaMKII is stimulated by MEL at 10−7 and 10−9 M, which is a concentration reached during the night in cerebrospinal fluid. However, under the same conditions but in an aqueous microenvironment, the activity of CaMKII was diminished (Argueta et al., 2022). Thus, evidence suggests that in addition CaM recruitment and enrichment at specific microdomains, MEL will down- or upregulate Ca2+-CaM activity, and, in consequence, the activity of its target enzymes, such as CaMKII, according to the microenvironment (lipid or aqueous).
Calmodulin Oxidation: Repercussion on its Capability to Activate Enzyme Targets
Ca2+-CaM is susceptible to oxidation due to the increase in calcium concentrations. Leading to elevated levels of reactive oxygen species. Transitory calcium concentrations within the cells can be caused by entrance through membrane channels or by its release from deposits in the endoplasmic reticulum (Peng and Jou, 2010).
CaM has in its primary sequence nine methionines, two prolines, seven lysines, six arginines, and 12 threonines. All of them can be susceptible to S-oxidation by sulfoxydation or carbonylation reactions (Gao et al., 1998; McCarthy et al., 2015). Current evidence indicates that oxidation of CaM can diminish its functions (Bartlett et al., 2003). When methionine 144 and 145 are oxidized, CaM loses its calcium binding capability due to the incorporation of sulfoxide or carbonyl group (Gao et al., 1998) and a conformational structural change occurs. Moreover, increased CaM oxidation could derive in ubiquitination and degradation (Drazic and Winter, 2014) deteriorating CaM dependent functions, (Cui et al., 2012).
Importantly, oxidation of CaM causes this protein to lose its capability to activate its target enzymes. CaMKII (Robison et al., 2007), calcineurin (Carruthers and Stemmer, 2008), and some receptors of the immune response, such as the Fas-associated death domain and tumor necrosis factor receptor type 1-associated death domain (Papoff et al., 2015), among others, are affected for this specific oxidation.
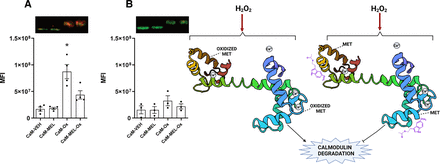
We do not know the time window for the effect of MEL as a CaM antagonist. However, preliminary experiments suggest that MEL antagonism of CaM last for up to 5 minutes. Thus, it is tempting to speculate that if oxidized MEL dissociates from CaM, then it means that it has a short-time effect as an antagonist. Recent experiments in vitro indicate that MEL antagonizes the action of CaM in a lapse of minutes, thus a transient antagonism might occur (Argueta et al., 2022). After MEL dissociation from CaM, MEL may first induce phosphorylation of this protein through PKC stimulation, and then its recruitment at specific microdomains (Soto-Vega et al., 2004). Moreover, selective oxidation of methionine residues of CaM causes a rapid decrease in the binding of radiolabeled CaM antagonists, such as trifluoperazine, W7, and chlorpromazine (Tanaka et al., 1983). Experiments done in our laboratory showed that MEL at 10−7 M blocks in vitro the Ca2+-CaM oxidation. A decrease of 50% in the amount of carbonylated CaM was observed in presence of hydrogen peroxide and MEL. By contrast, CaM was oxidized in presence of MEL, and the calcium chelator, EGTA, similarly to the control incubated only with the vehicle (Fig. 6). For these reasons and considering that MEL binds in a similar binding site of CaM antagonists, it is possible that MEL can be oxidized to protect methionine against carbonylation and the activity and structural conformation of CaM.
Effects of melatonin binding to Ca2+-CaM on oxidation. CaM was incubated with 10 mM H2O2 and the relative levels of carbonylated CaM was determined by Western blot (A, B). Oxidized levels of CaM were determined in the dumbbell CaM conformation adopted by CaM in presence of calcium (0.5 mM) by measuring carbonylated CaM showed in red (A) and in a random conformation of CaM adopted in presence of the calcium chelator EGTA (0.5 mM). (B) Scarce carbonylated CaM is shown in red, and non-oxidized CaM in green. The graph shows Median Fluorescence Intensity of carbonylated CaM incubated with either the vehicle (CaM-VEH), with 10−7 M melatonin (CaM-MEL), and in presence of 10 mM H2O2 and the vehicle (CaM-Ox) or 10 mM H2O2 and melatonin 10−7 M (CaM-MEL-Ox). Data are the mean of one experiment done by quadruplicate and repeated three times with similar results. Statistical analyses were done by two-way ANOVA followed by Tukeýs. Asterisk indicates a significant difference between CaM-Ox and CaM-MEL-Ox, *, P = 0.04.
Representative scheme shows in the left side melatonin binding to CaM in presence of calcium and the relative positions of methionine (MET) located in the hydrophobic pocket of CaM in the EF-hand domains (dumbbell conformations). CaM is represented in an oxidant and non-oxidant conditions with and without of melatonin represented by its chemical structure (purple).
Remarkably, antioxidants such as Trolox, a derivative of vitamin E which binds to human serum albumin, has a better antioxidant protector effect than other antioxidants because it has a dual role: it acts as a free radical scavenger and produces a shielding effect through protein binding (Salvi et al., 2001). In this regard, CaM has two methionines exposed in the hydrophobic pocket formed by adoption of an EF hand structure. Thus, it is possible that binding of MEL to these hydrophobic pockets may prevent the oxidation of the exposed methionines through a shielding effect, preserving the functionality of CaM to activate its multiple target enzymes. However, more experiments are necessary to demonstrate if MEL has a shielding effect on CaM oxidation.
Conclusions
The evidence presented in this review supports that MEL binding to CaM is an ancient phylogenetically conserved biochemical process that allows unicellular organisms to adapt to environmental conditions. MEL also blocks the oxidation of CaM’s methionines, which are exposed when this protein adopts a dumbbell shape by calcium binding. This protection against oxidation conserved the functionality of CaM. In multicellular organisms, MEL modulation of CaM functions prompts neurodevelopment, which is an essential function for the adaptation of organisms to their environment.
MEL has dual effects on CaM activity, acting as an antagonist or stimulating the activity of Ca2+-CaM target proteins. We can explain these dual effects of MEL due to the time of MEL interaction with CaM to produce CaM antagonism (Argueta et al., 2022), the recruitment of CaM at specific subcellular compartments or microdomains (Antón-Tay et al., 1998; Soto-Vega et al., 2004), and the microenvironment (aqueous or lipidic) (Argueta et al., 2022) where MEL and CaM interact. Evidence supports that those dual effects of MEL on CaM activity has physiologic consequences, such as modulation of cytoskeletal organization, cell proliferation, and neurodevelopment.
Finally, more research is necessary to understand how MEL may modulate calcium signaling and its intracellular concentrations and the physiologic meaning of these actions.
Acknowledgments
Figure 3 was modified with permission from Elíeset al., (2020) originally published in An Update to Calcium Binding Proteins. In: Islam, M. (eds) Calcium Signaling. Advances in Experimental Medicine and Biology. Springer Nature License No 5776850380329.
The authors thank Jorge Estrada Benítez and Itzel de Aquino for English corrections.
Data Availability
This article contains no datasets generated or analyzed during the current study.
Authorship Contributions
Participated in research design: Benítez-King, Argueta, Miranda-Riestra, Estrada-Reyes.
Conducted experiments: Argueta, Miranda-Riestra, Estrada-Reyes.
Performed data analysis: Benítez-King, Argueta, Miranda-Riestra, Muñoz-Delgado, Estrada-Reyes.
Wrote or contributed to the writing of the manuscript: Benítez-King, Muñoz-Delgado, Estrada-Reyes.
Footnotes
- Received October 12, 2023.
- Accepted April 15, 2024.
This work was supported by The National Institute of Psychiatry “Ramón de la Fuente Muñiz” [Grant IC22173.0].
The authors declare that they do not have conflicts of interest with the contents of this article.
In memoriam: Professor Fernando Antón-Tay.
Abbreviations
- CaM
- calmodulin
- CaMKII
- calmodulin-dependent protein kinase II
- MEL
- melatonin
- NMR
- nuclear magnetic resonance spectroscopy
- PKC
- protein kinase C
- W7
- N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride
- Copyright © 2024 by The Author(s)
This is an open access article distributed under the CC BY-NC Attribution 4.0 International license.