In the above article [Sun ZJ, Chen G, Zhang W, Hu X, Liu Y, Zhou Q, Zhu LX, and Zhao YF (2010) Mol Pharmacol 79:106–118], Fig. 3 and 4 were incorrect because of errors during proof processing. The corrected figures appear below.
The online version of this article has been corrected in departure from the print version.
The printer regrets this error and apologizes for any confusion or inconvenience it may have caused.
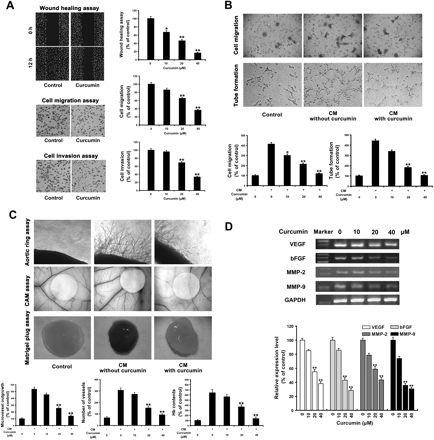
Curcumin inhibits the ability of ACC cells to migrate/invade and to induce angiogenesis in vitro and in vivo. For cell migration/invasion detection, ACC-M cells were treated with or without the indicated concentrations of curcumin for 12 h. For angiogenesis assays, ACC cells were further incubated with fresh serum-deprived medium in the absence of curcumin for another 24 h, followed by the collection of CM. A, inhibitory effects of curcumin on ACC cell migration and invasion were determined by the wound-healing migration assay and the Transwell Boyden chamber with polycarbonate filters (8 μm pore size) coated without or with Matrigel, respectively. Migrated or invaded cells were counted and analyzed after a 12-h exposure to DMSO (Control) or indicated concentrations of curcumin. Columns, mean from three independent experiments; bars, ± S.E. *, P < 0.05; **, P < 0.01 versus the control group. B and C, CM-induced in vitro migration and capillary network formation of EAhy926 cells (B) and CM-induced in vivo angiogenesis in the rat aortic ring, CAM, and mouse Matrigel plug assays (C). Control, a blank medium was added; in the other two, CM without or with curcumin pretreatment was added, as indicated. The percentage of inhibition was expressed using blank group as 100% (bottom). Columns, mean from three independent experiments; bars, ± S.E. *, P < 0.05; **, P < 0.01 versus the CM without curcumin treatment group. D, the mRNA expression (top) and protein secretion (bottom) levels of VEGF, bFGF, MMP-2, and MMP-9 by ACC-M cells were determined by RT-PCR and ELISA, respectively, after a 12-h exposure to DMSO (Control) or indicated concentrations of curcumin. All data are presented as the mean from three different experiments with duplicate (*, P < 0.05; **, P < 0.01 versus the control group); bars, ± S.E.
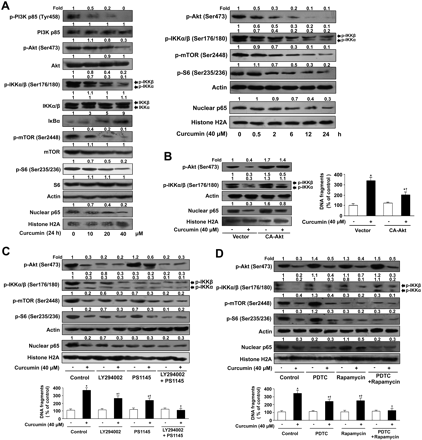
Curcumin dually inhibits the mTOR and NF-κB pathways through a crossed PI3K/Akt/IKK signaling axis. A, ACC-M cells were treated with indicated concentrations of curcumin in serum-deprived medium for indicated time. The expression levels of PI3K, Akt, IKKα/β, mTOR, S6, and their phosphorylation forms, as well as IκBα and nuclear form of NF-κB were determined by Western blotting. B, ACC-M cells were transfected with CA-Akt and vector plasmids, followed by incubation with curcumin (40 μM) in serum-deprived medium for 24 h. The expression levels of p-Akt, p-IKK, and nuclear NF-κB were detected by Western blotting. C, ACC-M cells were pretreated with LY294002 (20 μM) or/and PS1145 (10 μM) for 1 h followed by incubation with curcumin (40 μM) in serum-deprived medium for 24 h. The expression levels of the phosphor-form of mTOR, S6, IKK, and Akt, as well as the nuclear form of NF-κB, were analyzed by Western blotting. D, ACC-M cells were pretreated with PDTC (20 μM) or/and rapamycin (100 nM) for 1 h, followed by incubation with curcumin (40 μM) in serum-deprived medium for 24 h. The expression levels of p-mTOR, p-S6, p-IKK and p-Akt, as well as nuclear NF-κB, were measured by Western blotting. Densitometric quantitation relative to the control is shown on the top of the immunoreactive bands. Cell apoptosis was assessed by quantitation of DNA fragmentation using Cell Death Detection ELISAPLUS kit. The percentage of DNA fragmentation was calculated by considering 100% fragmentation unitedly at the time of curcumin addition. All data are presented as the mean from three different experiments with duplicate (*, P < 0.01 versus DMSO-treated control cells; †, P < 0.01 versus curcumin-treated cells without any other pretreatment); bars, ± S.E.
- Copyright © 2011 The American Society for Pharmacology and Experimental Therapeutics