Abstract
Representational difference analysis was used to isolate cDNAs corresponding to 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin)-inducible genes from mouse Hepa-1 cells. One cDNA encoded a novel cytochrome P450. The human homolog was also isolated and later proved to be human CYP2S1. The induction of mouse CYP2S1 mRNA by dioxin represents a primary response and required the aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator proteins. The induction of CYP2S1 also occurred in mouse liver and lung, with the highest expression found in lung. CYP2S1 was also inducible in a human lung epithelial cell line. The dioxin-inducibility of CY2S1 is exceptional, because all previously well-characterized cases of the induction of cytochromes P450 by dioxin involve members of the CYP1 family.
The cytochrome P450 (P450) superfamily of proteins is involved in the metabolism of a vast array of carcinogens, drugs, and endogenous metabolites. The three members of the CYP1 cytochrome P450 family are inducible by ligands for the aryl hydrocarbon receptor, which include important classes of chemical carcinogens such as PAHs, aromatic amines, and 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin). All three CYP1 family members also play important roles in the metabolic activation of a number of these same compounds to carcinogenic derivatives. CYP1A1 and CYP1B1 both activate PAHs to carcinogenic intermediates, whereas CYP1A2 activates aromatic amines. Dioxin is the most carcinogenic substance evaluated (Hankinson, 1995). In contrast to PAHs and aromatic amines, dioxin is refractory to biotransformation, and the parent compound itself acts as a carcinogen. The induction of CYP1A1 has been well-studied. After binding the ligand, the AHR translocates to the nucleus, where it dimerizes with the ARNT protein. The AHR/ARNT dimer then binds to xenobiotic-responsive elements in the 5′ flanking region of theCYP1A1 gene, leading to stimulation of transcription of the gene (Whitlock, 1999). Transcriptional activation of the CYP1A2 and CYP1B1 genes seems to occur in a similar fashion. Induction of CYP1A2 is restricted to the liver, whereas CYP1A1 and CYP1B1 are both inducible in many tissues (Savas et al., 1994; Dey et al., 1999).
The aim of this study was to identify novel dioxin-inducible genes, with the notion that such genes might be involved in carcinogenesis by PAHs, dioxin, and related compounds. Using representational difference analysis (RDA) on the mouse hepatoma cell line Hepa-1, we identified several genes not known previously to be inducible by dioxin. This report focuses on one of these genes, a novel cytochrome P450 that belongs to the CYP2 family rather than to the CYP1 family, which includes all other previously well-characterized, dioxin-inducible cytochromes P450.
Materials and Methods
Chemicals and Reagents.
Dioxin was obtained from the National Cancer Institute Chemical Carcinogen Repository (Bethesda, MD). Dimethyl sulfoxide (DMSO) and cycloheximide were purchased from Sigma-Aldrich (St. Louis, MO).
Cells and Cell Culture.
Mutant strains of the mouse hepatoma cell line Hepa1c1c7 (Hepa-1) that are deficient in ARNT (c4) and AHR (c12) were constructed in this laboratory previously (Hankinson, 1994), as was the mutant that expresses a defective AHR that is impaired in XRE binding activity (c35) (Sun et al., 1997). A549 (human lung carcinoma), MCF-7 (human mammary adenocarcinoma), HepG2 (human hepatocellular carcinoma), and Hep3B (human hepatocellular carcinoma) cell lines were obtained from the American Type Culture Collection (Manassas, VA). All of the cell lines were cultured in nucleoside-free α-minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Omega, Tarzana, CA), 100 U/ml penicillin, 100 μg/ml streptomycin (Gemini Bio-Products, Woodland, CA), and 0.25 U/ml amphotericin (Omega). Cells were treated when they had reached 70% confluence.
Mice.
C57BL/6 female mice at 6 weeks of age were obtained from Jackson Laboratories (Bar Harbor, ME). They were injected intraperitoneally with dioxin or solvent control (DMSO) and sacrificed 24 h later. Injection and dissection of animals were performed in a hood in a “carcinogen suite,” maintained under negative pressure and located in the UCLA Medical school vivarium. Exposed mice were housed in this same suite.
Representational Difference Analysis.
Representational difference analysis is a method for identifying differentially expressed genes with the use of several cycles of subtractive hybridization followed by PCR amplification. Details of the procedure will be described in a future publication. Briefly, Hepa-1 cells were exposed to either 10 nM dioxin in DMSO or DMSO alone for 24 h. mRNA was isolated from cells using the FastTrack 2.0 mRNA isolation kit according to the manufacturer's instructions (Invitrogen). Double-stranded cDNA was synthesized from mRNA using the standard techniques. RDA was performed as described previously (Chang and Denny, 1998). At the completion of four cycles, the difference product was randomly cloned into the pTarget cloning vector (Promega, Madison, WI). Clones were screened for dioxin inducibility using a modified reverse Northern blot technique whereby clones were screened by hybridization with randomly [32P]dATP-labeled cDNA (Prime-A-Gene Labeling Kit; Promega) from treated and untreated cells. The induction of potentially up-regulated clones was confirmed by Northern blot analysis, and positive clones were sequenced subsequently using an Applied Biosystems PRISM cycle-sequencing system (Laragen Inc., Santa Monica, CA).
5′ Rapid Amplification of cDNA Ends.
To identify the isolated cDNA fragments discovered by the RDA, a BLAST search in the NCBI database (http://www.ncbi.nlm.nih.gov) was performed. One of the clones did not match any of the previously cloned genes/cDNAs in the NCBI database. However, a search on the EST databases performed with the RDA fragment revealed a series of contiguously overlapping EST fragments, some of which possessed homology to C-terminal sequences of known P450 enzymes. A subsequent comparison of these mouse EST fragments with human ESTs revealed the presence of homologous human fragments. To clone the N-terminal 916 base pairs of the mouse cDNA and the N-terminal 1052 base pairs of the human cDNA that were not available in the EST databases, we used the SMART RACE kit and Advantage-GC 2 polymerase mix (CLONTECH, Palo Alto, CA). The RACE primers (5′-gta tct cag cag gag cag gag ggc ata g-3′ for the mouse and 5′-tca tgc aga acc gcg tcg gtg taa g-3′ for the human sequence) were designed using EST sequence information. As a template, we used reverse-transcribed mRNA from Hepa-1 or A549 cells treated with dioxin for 24 h. Sequence information obtained from the RACE products was subsequently used to amplify the full-length mouse and human coding regions, which were then cloned into the pTarget vector and sequenced.
Northern Analysis.
Cells were exposed to 10 nM dioxin (100 nM for human cell lines) in DMSO or DMSO alone for the periods indicated. C57BL/6 mice were injected with 30 μg/kg dioxin or vehicle and killed at the indicated times. mRNA was isolated using the Fast Track 2.0 mRNA isolation kit according to the manufacturer's instructions (Invitrogen). Protein synthesis inhibition was effected by cycloheximide (100 μM). Northern blot analysis was performed according to standard protocols. Full-length mouse and human CYP2S1 cDNAs were labeled by random primed [32P]dATP incorporation (Prime-A-Gene Labeling Kit; Promega). Phosphorimaging analysis was performed by the use of a 455SI PhosphorImager (Molecular Dynamics, Sunnyvale, CA) with correction for interlane load variability by comparison with ChoB, a constitutively expressed gene that is not responsive to dioxin or cycloheximide. ChoB cDNA was kindly provided by Dr. Harvey Herschman (UCLA, Los Angeles, CA).
In Vitro Transcription/Translation.
In vitro transcription/translation was performed using PCR products of the mouse and human CYP2S1 full-length coding regions. To allow transcription from the PCR product, 5′ primers containing the T7 promoter sequence were designed. Expression was achieved using the TnT T7-coupled reticulocyte lysate system according to the manufacturer's instructions (Promega) in the presence of [35S]methionine (ICN, Costa Mesa, CA). Protein products were analyzed on a 10% SDS-polyacrylamide gel, and the size of the protein was determined by comparison with a standard size marker (Bio-Rad, Hercules, CA).
Results
Isolation of a Novel Dioxin-Inducible Cytochrome P450.
An RDA experiment performed on the Hepa-1 cell line comparing cDNAs from untreated cells and cells treated for 24 h with dioxin led to the isolation of several dioxin-inducible clones. This report focuses on one of these clones. Databank searches with the sequence of this RDA clone revealed a series of contiguously overlapping ESTs. The assembled sequence for this set of ESTs indicated that this clone represented a previously uncloned cytochrome P450. Further databank analyses also indicated the existence of a human homolog for this gene. Because the sequence data available in the EST database covered only the C-terminal end of these putative new P450s, 5′-RACE was used to obtain the full-length coding sequence of both the mouse and human cDNAs. The human cDNA we identified proved later to be identical with human CYP2S1 (Rylander et al., 2001). We therefore called our mouse and human homologs CYP2S1. Alignment of human and mouse CYP2S1 amino acid sequences showed that the proteins are 78% identical (Fig.1). The cDNAs are 81% identical in nucleotide sequence. Mouse CYP2S1 exhibits 49% identity with mouse cytochromes CYP2G1 and CYP2B10, whereas the closest relatives of human CYP2S1 are members of the CYP2A and CYP2B subfamilies (CYP2A6, CYP2A13, and CYP2B6), which show 49% homology with CYP2S1.
Alignment of mouse and human CYP2S1 amino acid sequences. The mouse CYP2S1 cDNA codes for 501 and human CYP2S1 for 504 amino acids. The human and mouse amino acid sequences exhibited 78% identity. +, an amino acid of similar biochemical properties. The nucleic acid sequence of the mouse CYP2S1 cDNA has been sent to NCBI.
Full-length cDNAs for both mouse and human CYP2S1 were generated by PCR downstream from a T7 promoter sequence and expressed by in vitro transcription/translation. The proteins generated were both 56 kDa compared with standard-size markers (data not shown). This is the appropriate size predicted from the amino acid sequences of the two proteins.
Characterization of mCYP2S1 Induction by Dioxin in Mouse Cells.
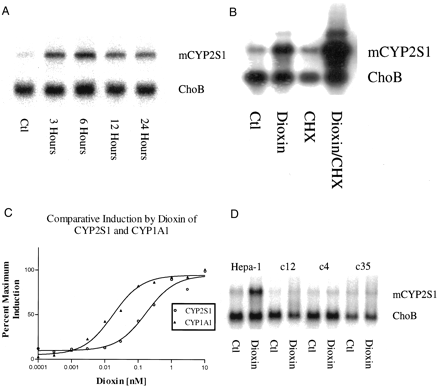
The mouse CYP2S1 mRNA detected in Hepa-1 cells is approximately 2.6 kb. Induction of this mouse CYP2S1 mRNA by 10 nM dioxin in Hepa-1 cells was evident in 3 h, was maximal at 6 h, and declined thereafter (Fig. 2A). The average maximal induction was 10-fold. Induction by dioxin was not blocked by cycloheximide (a protein-synthesis inhibitor) at a concentration 10-fold greater than that known to inhibit protein synthesis in Hepa-1 cells by 95 to 97% (Israel and Whitlock, 1983). This indicates that dioxin induction of mCYP2S1 is a primary response. Cycloheximide treatment resulted in superinduction of mCYP2S1, a phenomenon that also occurs for the dioxin-inducible CYP1A1 gene (Israel et al., 1985) (Fig. 2B). Dose-response analysis for the induction of CYP2S1 and CYP1A1 mRNAs in Hepa-1 cells demonstrated that the EC50 for the induction of CYP2S1 (0.17 nM) by dioxin is 10-fold greater than that for CYP1A1 (0.017 nM) (Fig. 2C). No induction of CYP2S1 in response to dioxin occurred in AHR- and ARNT-deficient derivatives of Hepa-1 cells, indicating that the induction of mouse CYP2S1 requires the presence of both AHR and ARNT (Fig. 2D).
Northern blotting analyses of mCYP2S1 expression in mouse Hepa-1 cells. A, time course of induction of mCYP2S1. Hepa-1 cells were treated with 10 nM dioxin for the indicated times or with vehicle (DMSO) only (Ctl). ChoB, a constitutively expressed gene that does not respond to dioxin or cycloheximide, was used to correct for interlane load variability. B, effect of cycloheximide on mCYP2S1 induction. Hepa-1 cells were treated with dioxin (10 nM), cycloheximide (CHX, 100 μM), or both for 6 h. C, dose-response curve for the induction of mCYP2S1 and mCYP1A1 by dioxin. Hepa-1 cells were treated with various concentrations of dioxin for 6 h, and mRNA was subjected to Northern blot analysis using CYP2S1, CYP1A1, and ChoB probes. CYP2S1 and CYP1A1 mRNA levels were quantitated by PhosphorImaging analysis and corrected for the amounts of ChoB mRNA. The figure represents the result from two independent experiments, except for the data for 0.0001 and 0.0003 nM dioxin, each of which were obtained from a single experiment. D, induction of mCYP2S1 in response to 10 nM dioxin in wild-type Hepa-1 cells and mutant cell lines deficient in expression of AHR (c12) or ARNT (c4) or in the mutant expressing AHR with impaired XRE binding activity (c35).
CYP2S1 Induction in Mice.
CYP2S1 mRNA induction occurred in the liver of C57BL/6 mice injected intraperitoneally with 30 μg/kg dioxin (Fig. 3A). The induction of CYP2S1 was most pronounced in the lung compared with induction in the liver, heart, kidney, and spleen (Fig. 3B). The Northern blot in Fig. 3B was exposed for a considerably shorter time than that for Fig. 3A so as not to overexpose the lanes containing the lung samples. Consequently, little or no signal was detectable for the liver samples in Fig. 3B.
Induction of CYP2S1 in mice treated with 30 μg/kg dioxin or vehicle (DMSO). A, induction in mouse liver 4, 8, and 12 h after treatment. B, induction in liver, lung, kidney, and heart tissues 24 h after treatment.
Expression of CYP2S1 in Human Cells.
The human lung cell line A549 exhibited much higher levels of the approximately 2.6-kb CYP2S1 mRNA than did the hepatoma cell lines HepG2 and Hep3B and the breast cancer cell line MCF-7. Dioxin treatment resulted in approximately a 2-fold induction of CYP2S1 mRNA in the A549 cell line (Fig.4A). The expression of CYP1A1 exhibited an inverse pattern of expression compared with CYP2S1 in these cell lines (Fig. 4A).
A, Induction of CYP2S1 and CYP1A1 in various human cell lines at 24 h in response to 100 nM dioxin.
Discussion
We describe a novel cytochrome P450 whose mRNA is inducible by dioxin. This cytochrome P450, CYP2S1, is exceptional in that other well-characterized dioxin-inducible P450s all belong to the CYP1 family, although hamster CYP2A8 has previously been shown to be inducible by PAHs in a XRE-dependent process and may therefore represent another dioxin-inducible CYP2 family member (Kurose et al., 1999).
The EC50 of dioxin for the induction of mCYP2S1 mRNA in Hepa-1 cells was 10-fold greater than that for CYP1A1 mRNA. The reason for this difference is not currently known, although it is clear from the results with the Hepa-1 mutant cell lines that, like CYP1A1, AHR and ARNT are required for the induction of CYP2S1. The EC50 for the induction of CYP1B1 by dioxin in rat liver has been shown to be 24-fold greater than that for CYP1A1 (Santostefano et al., 1997).
The level of induced m2S1 mRNA was much greater in the lung than in the other mouse tissues examined, including the liver. CYP2S1 mRNA was also found to be 2-fold inducible in the human lung cell line A549, whereas both uninduced and induced levels were low in the human hepatoma and breast cancer cell lines examined. Thus, although CYP2S1 mRNA was identified via its induction in mouse hepatoma cells, it seems to be expressed only weakly in normal mouse liver and human hepatoma cells. Among the limited set of human cell lines examined, a reciprocal relationship between CYP2S1 and CY1A1 inducibility was observed. It is of interest to examine additional human cell lines to determine whether there is one in which CYP2S1 is more dramatically inducible by dioxin.Rylander and coworkers (2001) recently reported that CYP2S1 is constitutively expressed in several human tissues, including the lung. Because CYP2S1 is inducible by dioxin, we speculate that, like the other known dioxin-inducible P450s, it is capable of metabolizing important classes of chemical carcinogens. In addition, because of the high levels of CYP2S1 in dioxin-treated mouse lung and in the human lung cell line A549, we also speculate that it may play an important role in carcinogen metabolism in the lung, a major target tissue for a number of environmental carcinogens, including tobacco smoke. Consequently, the identification of substrates for CYP2S1 is of considerable interest.
Acknowledgments
We thank Long Hung for his outstanding technical support and Drs. Christopher Denny, Harvey Herschman, and Linda Vician for their technical advice on RDA.
Footnotes
- Received September 14, 2001.
- Accepted October 30, 2001.
-
Supported by National Cancer Institute grant R01-CA28868, a Research Training Fellowship from the International Agency for Research on Cancer (S.T.S.), The Academy of Finland Grant 46590 (S.T.S.), The Finnish Work Environment Fund Grant 100389 (S.T.S.), and a Research Supplement to Underrepresented Minorities to 2-R01-CA28868–20 (S.P.R.)
-
S.P.R. and S.T.S. contributed equally to this work.
Abbreviations
- P450
- cytochrome P450
- PAH
- polycyclic aromatic hydrocarbon
- dioxin
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- RDA
- representational difference analysis
- DMSO
- dimethyl sulfoxide
- PCR
- polymerase chain reaction
- NCBI
- National Center for Biotechnology Information
- EST
- expressed sequencer tag
- RACE
- rapid amplification of cDNA ends
- Hepa-1
- hepatoma cell line Hepa1c1c7
- ChoB
- Chinese hamster ovary B
- AHR
- aryl hydrocarbon receptor
- ARNT
- aryl hydrocarbon nuclear translocator
- XRE
- xenobiotic-responsive element
- The American Society for Pharmacology and Experimental Therapeutics