Abstract
Biased agonism on the type I angiotensin receptor (AT1-R) can achieve different outcomes via activation of G protein–dependent and –independent cellular responses. In this study, we investigated whether the biased activation of AT1-R can lead to different regulation and intracellular processing of the receptor. We analyzed β-arrestin binding, endocytosis, and subsequent trafficking steps, such as early and late phases of recycling of AT1-R in human embryonic kidney 293 cells expressing wild-type or biased mutant receptors in response to different ligands. We used Renilla luciferase–tagged receptors and yellow fluorescent protein–tagged β-arrestin2, Rab5, Rab7, and Rab11 proteins in bioluminescence resonance energy transfer measurements to follow the fate of the receptor after stimulation. We found that not only is the signaling of the receptor different upon using selective ligands, but the fate within the cells is also determined by the type of the stimulation. β-arrestin binding and the internalization kinetics of the angiotensin II–stimulated AT1-R differed from those stimulated by the biased agonists. Similarly, angiotensin II–stimulated wild-type AT1-R showed differences compared with a biased mutant AT1-R (DRY/AAY AT1-R) with regards to β-arrestin binding and endocytosis. We found that the differences in the internalization kinetics of the receptor in response to biased agonist stimulation are due to the differences in plasma membrane phosphatidylinositol 4,5-bisphosphate depletion. Moreover, the stability of the β-arrestin binding is a major determinant of the later fate of the internalized AT1-R receptor.
Introduction
Ligand binding to receptors can initiate several parallel signal transduction pathways, leading to various final outcomes in the same cell. It has been recognized that several ligands are capable of selectively initiating distinct signal transduction pathways from the same receptor, leading to the concept of biased agonism. It is well accepted that biased agonism is an important feature of G protein–coupled receptors (GPCRs), and it is proposed that development of biased agonists can serve a new therapeutic approach (Whalen et al., 2011). The binding of agonists to GPCRs initiates the G protein–mediated pathway, which results in the production of second messengers. The downregulation of activated receptors involves several consecutive or parallel processes, such as desensitization, followed by internalization into vesicles and recycling or degradation. Receptor internalization is regulated by GPCR kinases (GRKs), with subsequent binding of β-arrestin (Lefkowitz, 2007). The β-arrestin binding not only mediates desensitization and internalization, but also triggers additional signal transduction pathways, which are often called G protein–independent signaling routes.
Angiotensin II (AngII) is the main effector of the renin-angiotensin system and can activate the type 1 (AT1-R) angiotensin receptor. After binding to AT1-R, the Gq/11 protein mediates the activation of phosphoinositide-specific phospholipase Cβ (PLCβ) and hydrolysis of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] (Hunyady and Catt, 2006). Binding of AngII also triggers events leading to regulation of AT1-R, such as desensitization, internalization, degradation, and recycling to the cell surface (Hunyady et al., 2000; Ferguson, 2001). Desensitization and internalization of AT1-R are regulated by phosphorylation by GRKs, which promote β-arrestin binding and additional dynamin-dependent steps (Anborgh et al., 2000; Qian et al., 2001). Although a considerable amount of knowledge has been gained in understanding the mechanisms involved in the biased agonism of GPCRs (Godin and Ferguson, 2012; Reiter et al., 2012), less information is available about the fate (intracellular processing) of the receptors after biased activation.
The Rab proteins are key players in vesicular transport mechanisms, with the individual isoforms marking distinct vesicles in the endocytic and exocytic compartments (Zerial and McBride, 2001). Internalization, recycling, and degradation of receptors involve different Rab proteins, such as Rab5, Rab7, and Rab11 (Seachrist and Ferguson, 2003). It was shown that Rab5, Rab7, and Rab11 work together to regulate the vesicular trafficking of AT1-R (Dale et al., 2004). We have shown in confocal microscopy studies using green fluorescent protein (GFP)–tagged AT1-R that AngII stimulates rapid translocation of the receptor to Rab5 early endosomes, and subsequently to juxtanuclear Rab11 vesicles (Hunyady et al., 2002).
Studies of the G protein–independent signaling of AT1-R have been accelerated by several approaches. Mutations within the highly conserved Asp125Arg126Tyr127 sequence of AT1-R (DRY/AAY mutation) abrogated G protein coupling, yet such receptors can initiate ERK1/2 activation (Wei et al., 2003; Szidonya et al., 2007; Hansen et al., 2008). The development and utilization of a biased AT1-R agonist, [Sar1,Ile4,Ile8]AngII (SII-AngII), further widened the possibilities to investigate G protein–independent mechanisms (Holloway et al., 2002). Stimulation of AT1-R by SII-AngII does not activate G proteins, but stimulates G protein–independent mechanisms and is also able to induce internalization of the receptor (Luttrell et al., 2001; Wei et al., 2003). SII-AngII is a widely used tool to study the biased agonism of AT1-R in spite of its relatively low affinity to AT1-R. Recently, new biased peptide ligands of AT1-R were discovered such as TRV120023 (Sar-Arg-Val-Tyr-Lys-His-Pro-Ala-OH) and TRV120027 (Sar-Arg-Val-Tyr-Ile-His-Pro-D-Ala-OH), which have higher receptor binding affinities than SII-AngII (Violin et al., 2010). These TRV peptides selectively activate the β-arrestin–mediated signaling pathway and possess beneficial effects on cardiac contractility and performance (Violin et al., 2010; Kim et al., 2012). The favorable properties and potential clinical applications of TRV120027 are further investigated in trials for the treatment of acute heart failure (Soergel et al., 2013; Violin et al., 2014).
We used a bioluminescence resonance energy transfer (BRET)–based approach to investigate the fate of the receptor in response to stimuli. We used fluorescently labeled constructs marking the molecular steps during internalization and luciferase-fused receptors in BRET measurements. Our results demonstrate that biased agonists or stimulation of a biased receptor with the native AngII (AT1-R–DRY/AAY) yield(s) different fates of the activated receptors in terms of β-arrestin binding, internalization, and appearance in various intracellular compartments. Our findings also suggest that the intracellular processing of AT1-R is dependent on the type of activation and affinity of ligand binding to AT1-R. Bias ligand–bound receptors are unable to couple to Gq proteins and activate PLCβ to show accelerated internalization due to a lack of PtdIns(4,5)P2 hydrolysis in the plasma membrane. In contrast, the degradation and recycling of the internalized receptor is mainly determined by the strength of β-arrestin binding.
Materials and Methods
Cell culture dishes and plates for BRET measurements were purchased from Greiner (Kremsmunster, Austria). Coelenterazine h was from either Invitrogen (Carlsbad, CA) or Regis Technologies (Morton Grove, IL). SII-AngII was purchased from Bachem AG (Bubendorf, Switzerland). The TRV120023 and TRV120027 peptides were synthesized by Proteogenix (Schiltigheim, France) to more than 98% purity. Wortmannin (Wm) was purchased from Calbiochem (San Diego, CA), PIK93 was obtained from Sigma-Aldrich (St. Louis, MO), and the A1 inhibitor was synthesized as described (Bojjireddy et al., 2014). Rapamycin was obtained from Merck (Darmstadt, Germany). Unless otherwise stated, all other chemicals and reagents were purchased from Sigma-Aldrich. The human embryonic kidney (HEK) 293 cells were from American Type Culture Collection (Manassas, VA).
DNA Constructs.
For the construction of yellow fluorescent protein (YFP)–labeled constructs, the plasmid backbones of eYFP-C1 or eYFP-N1 (Clontech, Mountain View, CA) were used. The cDNA of the eYFP-tagged β-arrestin2 (β-arrestin2–YFP) and Renilla luciferase (Rluc)–tagged AT1-Rs (AT1-R–Rluc and AT1-R–DRY/AAY–Rluc) were constructed as described previously (Turu et al., 2006; Balla et al., 2012). The eYFP-labeled full-length Rab5, Rab7, and Rab11 were constructed by replacing the eGFP-coding region with eYFP in the GFP-tagged constructs as described previously (Hunyady et al., 2002). The eYFP-tagged PLCδ1 pleckstrin homology (PH) domain was constructed as described previously (Varnai and Balla, 1998), whereas the PLCδ1-PH–super Renilla luciferase (Sluc) was constructed by replacing the eYFP coding region with sequence of super Renilla luciferase (Woo and von Arnim, 2008). The constructs of the rapamycin-inducible heterodimerization system (PM-FRB-mRFP and mRFP-FKBP-5ptase) were constructed as described previously (Toth et al., 2012). The expression vector of dominant-negative (DN) mutant GRK2 (K220M) was kindly provided by Dr. S. S. G. Ferguson (Ferguson et al., 1995).
Cell Culture and Transfection.
The experiments were performed on the HEK293 cell line. The cells were cultured in Dulbecco’s modified Eagle’s medium with Pen/Strep (Invitrogen) and 10% heat-inactivated fetal bovine serum in 5% CO2 at 37°C. For BRET experiments, the cells were cultured in plastic dishes, trypsinized prior to transfection, transiently transfected by using Lipofectamine 2000 (Invitrogen), and plated on poly-lysine pretreated white 96-well plates in 1 × 105 cells/well density for BRET measurements. The DNA amounts were 0.25 μg Rluc-containing construct/well and 0.0625–0.125 μg YFP-containing construct/well. The amount of Lipofectamine 2000 was 0.5 μl/well. For suspension of Ca2+ measurements and the mitogen-activated protein kinase assay, the HEK293 cells were cultured in 10-cm dishes and six-well plates, respectively. The cells were transiently transfected with Lipofectamine 2000 according to the manufacturer’s protocol.
BRET Measurement.
We used a Renilla luciferase–fused receptor as the energy donor and an eYFP-tagged protein as the acceptor. The BRET measurements were performed after 24 hours of the transfection on white 96-well plates. The medium of the cells was changed prior to measurements to a modified Krebs-Ringer buffer containing 120 mM NaCl, 4.7 mM KCl, 1.8 mM CaCl2, 0.7 mM MgSO4, 10 mM glucose, and Na-HEPES 10 mM, pH 7.4, and the BRET measurements were performed at 37°C. The BRET measurements were started after addition of the cell-permeable substrate coelenterazine h (Invitrogen or Regis Technologies) at a final concentration of 5 μM, and the counts were recorded by using either Berthold Mithras LB 940 (Bad Wildbad, Germany) or Varioskan Flash (Thermo Scientific, Waltham, MA) readers that allow the detection of signals using filters at 485- and 530-nm wavelengths. The detection time was 0.25–0.5 seconds. The BRET ratios were calculated as a 530/485 nm ratio. Measurements were done in triplicates. The plotted BRET curves are the average of at least three independent experiments. BRET ratios were baseline corrected to the vehicle curve using GraphPad Prism software (La Jolla, CA). The approximate BRET ratio using the cytosolic eYFP and AT1-R–Rluc pair is ∼0.8 (data not shown).
Cytoplasmic Ca2+ Measurements in Cell Suspensions.
HEK293 cells were transfected with the various constructs (6 μg DNA/10-cm dish) by using Lipofectamine 2000. After 24 hours, the cells grown on 10-cm culture plates were removed by mild trypsinization, and aliquots of cells (∼106 cells) were loaded with 2 μM Fura-2/acetoxymethyl ester in Dulbecco’s modified Eagle’s medium containing 1.2 mM CaCl2, 3.6 mM KCl, and 25 mM HEPES containing 1 mg/ml bovine serum albumin, 0.06% Pluronic acid, and 200 μM sulfinpyrazone for 45 minutes at room temperature. Cells were then washed with the same medium without Fura-2/acetoxymethyl ester and stored at room temperature in the dark. The cells were centrifuged rapidly before the measurements and dispersed in 3 ml of the modified Krebs-Ringer buffer used for all other analysis. Calcium measurements were performed at room temperature in a PTI Deltascan spectrofluorometer (Photon Technology International, Princeton, NJ).
ERK1/2 Mitogen-Activated Protein Kinase Assay.
Twenty-four hours after transfection, the HEK293 cells were serum starved for 4 hours and stimulated for 5 minutes with 100 nM AngII or 10 μM SII-AngII. Cells were scraped into an SDS sample buffer containing protease and phosphatase inhibitors, briefly sonicated, boiled, and separated on SDS-polyacrilamide gels. The proteins were transferred to polyvinylidene fluoride membranes and incubated with the appropriate primary (Santa Cruz Biotechnology, Santa Cruz, CA) and secondary (Cell Signaling, Danvers, MA) antibodies. The antibodies were visualized by enhanced chemiluminescence using Immobilion Western HRP substrate reagents (Millipore, Billerica, MA).
Confocal Microscopy.
AT1-R–GFP stably expressing HEK293 cells (Hunyady et al., 2002) was plated on polylysine-pretreated glass coverslips (3 × 105 cells/35-mm dish). After 24 hours, the localization and distribution of the receptor were analyzed using a Zeiss LSM 710 confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany). Postacquisition data analysis of the confocal images was performed with either ZEN (Carl Zeiss) or MetaMorph (Molecular Devices, Sunnyvale, CA) software. Quantification of the internalization of AT1-R–GFP (location in intracellular vesicles, which are clearly separated from the plasma membrane) as a function of time was calculated from a morphometric analysis by MetaMorph software obtained in seven separate experiments.
Results
BRET Assay for the Detection of Agonist-Induced Internalization of AT1-R.
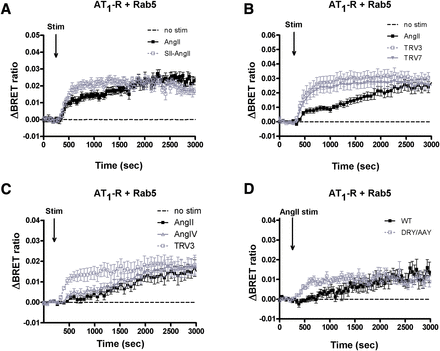
Our previous results raised the possibility that biased stimulation of AT1-R can lead to different fates of the internalized receptor (Balla et al., 2012). To investigate this question in more detail, we analyzed the proximity of the receptor with different Rab proteins used as markers of the steps along the endocytotic/recycling/degradation pathways of the internalized receptors. Rab5-YFP is a marker for early endosomes and Rab7-YFP is that for the multivesicular body/late endosome, whereas Rab11-YFP marks the late recycling route (Zerial and McBride, 2001). As shown in Fig. 1A, we detected an elevated BRET ratio between AT1-R–Rluc and Rab5-YFP after both AngII and SII-AngII stimulation (black filled and gray open symbols, respectively), which indicates receptor-mediated endocytosis. In our previous studies (Balla et al., 2012), we noted that at the early phase after stimulation, SII-AngII induced a more robust colocalization of the receptor with Rab5 endosomes compared with AngII stimulation (Fig. 1A, gray open symbols; also in Supplemental Fig. 6C). Therefore, we investigated the effects of newly developed biased agonists on the internalization and sequential intracellular trafficking steps of AT1-R. Figure 1B shows that stimulation with both 1 μM TRV120023 (TRV3) and 1 μM TRV120027 (TRV7) yielded BRET curves between the receptor and Rab5 that were very reminiscent of those evoked by SII-AngII. Taken together, the results shown in Fig. 1, A and B suggest that biased stimulation apparently accelerated the internalization of AT1-R. Since both SII-AngII and the TRV compounds have a lower affinity than AngII, we also tested a low affinity, but unbiased agonist, the hexapeptide angiotensin IV (AngIV) (Le et al., 2002) on the internalization rate of AT1-R. In contrast to the biased agonists, AngIV stimulation evoked a very similar BRET interaction of the receptor with Rab5 containing endosomes as the AngII-initiated response (Fig. 1C).
BRET assay between wild-type AT1-R and Rab5 upon stimulation (Stim) in HEK293 cells. (A–C) HEK293 cells were transfected with the plasmids of AT1-R–Rluc and YFP-fused Rab5 protein, and after 24 hours, the cells were exposed to either 100 nM AngII (black-filled squares), (A) 10 μM SII-AngII (gray open symbols), (B) 1 μM TRV120023 (TRV3; gray open squares) or 1 μM TRV120027 (TRV7; gray-filled triangles); (C) 10 μM AngIV (gray open triangles) or 1 μM TRV3 (gray open squares), or vehicle (dashed lines) at the indicated time points. (D) HEK293 cells were transfected with the plasmids of the indicated receptor Rluc (WT, wild type, black-filled symbols; DRY/AAY mutant, gray open symbols) and Rab5-YFP, and after 24 hours, the cells were exposed to either 100 nM AngII or vehicle (dashed line) at the indicated time points. The BRET records are the average of at least three independent experiments. Mean values ± S.E.M. are shown (n = 3).
It is well established that AT1-R–induced Gq activation causes PLC-mediated hydrolysis of the membrane PtdIns(4,5)P2, which can be monitored by following the translocation of PLCδ1-PH-GFP from the plasma membrane to the cytosol (Varnai and Balla, 1998). We confirmed that AngIV at the concentration used (10 μM) is able to initiate Gq activation and PtdIns(4,5)P2 hydrolysis in BRET measurements. AngIV, similarly to AngII, decreased the BRET ratio between the PLCδ1-PH domains fused with YFP and Sluc. When the PH domains are bound to the plasma membrane, they are within BRET distance, whereas being in the cytoplasm, they are not; therefore, the decreased BRET signal reflects the dissociation of the PtdIns(4,5)P2 sensor domains from the plasma membrane due to the breakdown of PtdIns(4,5)P2. The lipid is slowly resynthesized, causing the reappearance of the probes in the plasma membrane (BRET ratio increase) (Supplemental Fig. 1). It has been well documented that AngII stimulation of the DRY/AAY mutant receptor leads to biased activation, similar to that seen during SII-AngII stimulation of wild-type receptors (Wei et al., 2003). Figure 1D shows that AngII stimulation of the AT1-R–DRY/AAY–Rluc caused a similar BRET interaction with Rab5-YFP as the biased ligand stimulation of the wild-type receptor (Fig. 1, A and B). This result suggested that the AngII-induced internalization of the mutant receptor occurs faster than with the wild-type AT1-R. To demonstrate that the properties of the luciferase-tagged receptors are similar to those of the untagged receptors, we analyzed calcium signaling and extracellular signal-regulated kinase activation. The results showed that both of these responses were comparable with the untagged and Rluc-tagged receptors and that neither SII-AngII nor the DRY/AAY receptor stimulated by AngII showed Ca2+ responses, yet they displayed a reduced but still detectable ERK1/2 activation (Supplemental Fig. 2). These data together suggested that the tagged receptors function similarly to the nontagged AT1-Rs.
Characterization of the Biased Agonist–Induced Internalization of AT1-R.
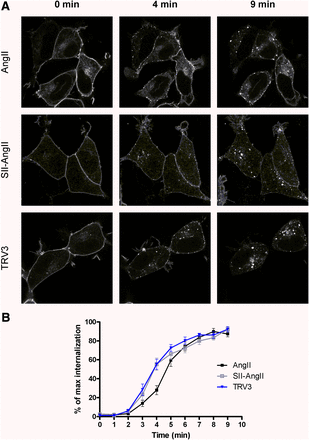
We also investigated the agonist-induced internalization of the receptor with confocal microscopy using HEK293 cells stably expressing AT1-R–GFP. Figure 2 demonstrates the slightly faster internalization of a biased-activated receptor. More receptors were detectable in intracellular vesicles after 4-minute stimulation with either SII-AngII or TRV120023 (TRV3) compared with AngII stimulation.
Effect of agonist stimulation on the distribution of AT1-R–GFP in HEK293 cells. (A) AT1-R–GFP stably expressing HEK293 cells were exposed to either 100 nM AngII (upper row of micrographs), 10 μM SII-AngII (middle row of micrographs), or 1 μM TRV120023 (TRV3; lower row of micrographs), and the GFP fluorescence was detected by a Zeiss LSM 710 confocal laser-scanning microscope. The representative confocal micrographs show the localization and cellular distribution of AT1-R before (0 minutes) and 4 or 9 minutes after agonist treatment. (B) Quantification of the internalization of AT1-R–GFP as a function of time, which was calculated from a morphometric analysis by MetaMorph software obtained in seven separate experiments (mean ± S.E.M.).
We then investigated the possibility that the more rapid internalization of the SII-AngII–stimulated receptor is the result of the use of an alternative endocytic pathway(s). We used 300 mM sucrose treatment to inhibit clathrin-mediated endocytosis (Heuser and Anderson, 1989). At this concentration (300 mM), sucrose significantly diminished the BRET ratio increase between the receptor and Rab5 after stimulation with either AngII or SII-AngII, which suggested that both ligands initiated a primarily clathrin-mediated endocytic mechanism (Supplemental Fig. 3, A and B). We also studied the effect of filipin, which is widely used for the inhibition of the clathrin-independent caveolae-mediated pathway (Orlandi and Fishman, 1998). During stimulation of the cells with 100 nM AngII or 10 μM SII-AngII, the preincubation of cells with filipin did not change the BRET ratio kinetic between Rab5 and AT1-R (Supplemental Fig. 3C).
Next, we analyzed whether the faster rate of Rab5 recruitment is the consequence of the absence of classic (G protein–dependent) calcium signaling in biased-stimulated AT1-R. We used BAPTA-AM pretreatment to blunt the evoked calcium signal. As shown in Supplemental Fig. 4A, BAPTA-AM pretreatment did not alter the kinetics of Rab5 recruitment. We confirmed that BAPTA-AM pretreatment was sufficient to inhibit the calcium signal in HEK293 cells (Supplemental Fig. 4B).
Effect of Biased Stimulation on the Association of AT1-R with β-Arrestin2.
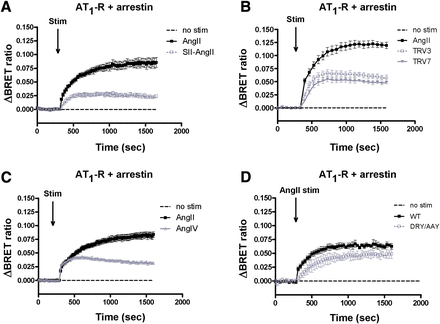
Next, we investigated the possible involvement of β-arrestin2 in the altered internalization of AT1-R when the receptor is activated by biased agonists. Both SII-AngII and the TRV compounds were able to cause β-arrestin2 recruitment, although the biased agonists evoked smaller responses than AngII stimulation (Fig. 3, A and B). It is noteworthy that the AngII-induced binding of β-arrestin2 appeared more sustained than that evoked by the biased agonists, (Fig. 3, A and B, gray symbols; also in Supplemental Fig. 6A, gray symbols). Here, AngIV caused a similar type of AT1-R–β-arrestin2 BRET interaction as the low-affinity biased agonists (Fig. 3C). We also measured complete concentration-response curves to check that the used agonist concentrations are maximally effective. Supplemental Figure 5 demonstrates that 100 nM of AngII induced maximal PtdIns(4,5)P2 hydrolysis (Supplemental Fig. 5A) and β-arrestin2 binding (Supplemental Fig. 5B). AngIV was maximally effective with a 10 μM concentration in PtdIns(4,5)P2 breakdown and β-arrestin2 recruitment assays (Supplemental Fig. 5, A and B). Ten micromolars of SII-AngII and 1 μM concentrations of TRV compounds evoked maximal β-arrestin binding (Supplemental Fig. 5B), but did not cause detectable PtdIns(4,5)P2 hydrolysis (Supplemental Fig. 1) or vasoconstriction in wire myography experiments using mouse aortic rings (data not shown).
BRET assay between AT1-R and β-arrestin2 upon agonist stimulation (Stim) in HEK293 cells. HEK293 cells were transfected with AT1-R–luciferase and β-arrestin2–YFP, and after 24 hours, the cells were exposed to 100 nM AngII (black-filled squares), (A) 10 μM SII-AngII (gray open symbols), (B) 1 μM TRV120023 (TRV3; gray open squares) or 1 μM TRV120027 (TRV7; gray-filled triangles), (C) 10 μM AngIV (gray open triangles), or vehicle (dashed lines) at the indicated time points. (D) HEK293 cells were transfected with the plasmids of the indicated receptor Rluc (WT, wild type, black-filled symbols; DRY/AAY mutant, gray open symbols) and β-arrestin2–YFP, and after 24 hours, the cells were exposed to either 100 nM AngII or vehicle (dashed line) at the indicated time points. The BRET records are the average of at least three independent experiments. Mean values ± S.E.M. are shown (n = 3).
The BRET curve of the AngII-induced β-arrestin binding of DRY/AAY–AT1-R is very reminiscent of that of the wild-type receptor, and the amplitude is ∼75% of the wild-type counterpart (Fig. 3D, black-filled symbols). We also compared the effects of stimulation of AT1-R–DRY/AAY–Rluc with SII-AngII and AngII. Stimulation of AT1-R–DRY/AAY–Rluc with SII-AngII resulted in slightly weaker and less stable β-arrestin binding than the AngII-induced response (Supplemental Fig. 6). Yet, the translocations to Rab5 endosomes of AT1-R–DRY/AAY–Rluc in response to either SII-AngII or AngII stimulation were almost identical (Supplemental Fig. 6).
Effect of Inhibitors of Phosphatidylinositol Kinases on the Agonist-Induced AT1-R Endocytosis.
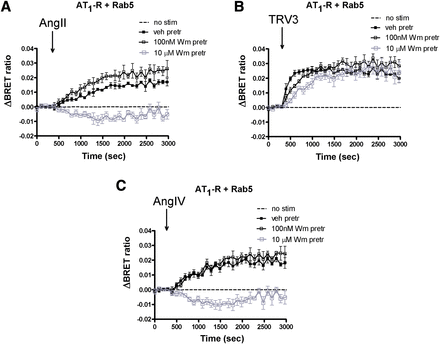
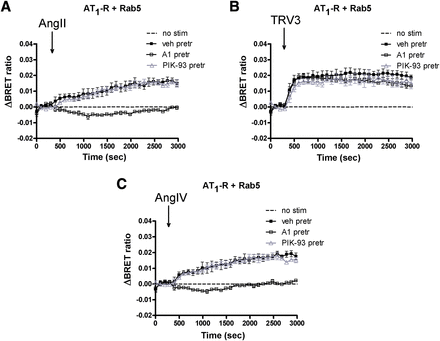
Next, we investigated the role of PtdIns(4,5)P2 in the regulation of AT1-R internalization. PtdIns(4,5)P2 is located on the inner leaflet of the plasma membrane and is important in both signal transduction processes and the endocytosis of cell surface receptors, including GPCRs (Toth et al., 2012). Several phosphatidylinositol kinases are involved in the maintenance of the plasma membrane PtdIns(4,5)P2 (Balla and Balla, 2006; Balla, 2013). Among these, phosphatidylinositol-4 kinases (PI4Ks) control the first step in PtdIns(4,5)P2 synthesis (Balla et al., 2005, 2008). First, we examined the effect of low and high concentrations of Wm (the former selectively inhibits most phosphatidylinositol-3 kinases; the latter also inhibits type III PI4Ks). Figure 4 shows that 100 nM Wm did not alter the agonist-induced endocytosis of AT1-R. In contrast, 10 μM Wm was able to diminish AngII- and AngIV-induced endocytosis (Fig. 4, A and C), but not the internalization evoked by the biased agonists (Fig. 4B). This result suggested the involvement of PI4Ks rather than phosphatidylinositol-3 kinases, and therefore we tested more selective inhibitors of the individual PI4K isoforms. PIK-93 inhibits PI4KIIIβ/PI4KB, but to a much lesser degree than PI4KA (Balla et al., 2008), whereas a compound, A1, inhibits PI4KIIIα/PI4KA more potently than PI4KB (Bojjireddy et al., 2014). Using these inhibitors and following the BRET signal between the receptor and Rab5, the activity of PI4KIIIα was found to be necessary for the AngII- and AngIV-induced endocytosis, but not for the biased agonist–induced response (Fig. 5), whereas PI4KIIIβ was found not to be responsible for the maintenance of PtdIns(4,5)P2 involved in the regulation of endocytosis.
Effect of wortmannin on the agonist-induced internalization of AT1-R in HEK293 cells. HEK293 cells were transfected with the plasmids of AT1-R–Rluc and Rab5-YFP proteins, and after 24 hours, the experiments were carried out. Cells were pretreated (pretr) for 10 minutes with vehicle (veh) BRET medium (black-filled symbols) or BRET medium supplemented with 100 nM wortmannin (black open symbols) or 10 μM wortmannin (gray open symbols) and exposed to either vehicle (dashed line), 100 nM AngII (A), 1 μM TRV120023 (TRV3) (B), or 10 μM AngIV (C) at the indicated time points. The BRET curves are the average of three independent experiments, each performed in triplicate. Mean values ± S.E.M. are shown (n = 3). Stim, stimulation.
Effect of A1 and PIK-93 on the agonist-induced internalization of AT1-R in HEK293 cells. HEK293 cells were transfected with the plasmids of the AT1-R–Rluc and Rab5-YFP proteins, and after 24 hours, the experiments were carried out. Cells were pretreated (pretr) for 10 minutes with vehicle (veh) BRET medium (black-filled symbols) or BRET medium supplemented with 10 nM A1 (black open squares) or 250 nM PIK-93 (gray open triangles) and exposed to either vehicle (dashed line), 100 nM AngII (A), 1 μM TRV120023 (TRV3) (B), or 10 μM AngIV (C) at the indicated time points. The BRET curves are the average of three independent experiments, each performed in triplicate. Mean values ± S.E.M. are shown (n = 3). Stim, stimulation.
Plasma Membrane PtdIns(4,5)P2 Depletion Affects AT1-R Internalization upon Unbiased or Biased Activation.
We also investigated the role of PtdIns(4,5)P2 in the stimulation-evoked Rab5 recruitment of AT1-R. First, we used a plasma membrane PtdIns(4,5)P2 depletion system, as described earlier (Toth et al., 2012). The appearance of AT1-R in early endosomes was followed by BRET measurements between AT1-R–Rluc and fluorescently tagged Rab5 in cells coexpressing the rapamycin-inducible PtdIns(4,5)P2 depletion system. Briefly, addition of rapamycin induces heterodimerization between the plasma membrane–targeted FRB and cytoplasmic FK506 binding protein; thus, the 5ptase domain is translocated to the plasma membrane, where it degrades PtdIns(4,5)P2. We confirmed the efficiency of this method in BRET measurements using YFP- and Sluc-tagged phospholipase Cδ1 PH domain sensors (data not shown). The depletion of plasma membrane PtdIns(4,5)P2 completely blocked both unbiased or biased activation–induced AT1-R Rab5 recruitment (Supplemental Figs. 6B and 7A).
We also performed experiments using a dominant-negative GRK2 construct to show the role of PtdIns(4,5)P2 in the regulation of AT1-R internalization. It was demonstrated that DN-GRK2 significantly blunts the receptor activation–induced PLCβ activity (Carman et al., 1999; Sallese et al., 2000), and we confirmed that overexpression of DN-GRK2 (GRK2-K220M) decreased the AT1-R stimulation–induced plasma membrane PtdIns(4,5)P2 hydrolysis in HEK293 cells (Supplemental Fig. 8A). Figure 6 shows that overexpression of DN-GRK2 accelerated AngII-evoked AT1-R internalization, but had no effect on the biased agonist (TRV120023)–induced response. The overexpression of DN-GRK2 did not affect the β-arrestin2 binding properties (Supplemental Fig. 8B), which supports that not only GRK2 but other GRK isoforms, such as GRK3/5/6, could be involved in receptor phosphorylation and β-arrestin2 recruitment in HEK293 cells (Kim et al., 2005).
Effects of attenuation of plasma membrane PtdIns(4,5)P2 hydrolysis on BRET curves between AT1-R and Rab5 or β-arrestin2. HEK293 cells were transfected with plasmids encoding DN-GRK2, AT1-R–Rluc, and Rab5-YFP. After 24 hours, the cells were exposed to either 100 nM AngII (red trace), 1 μM TRV120023 (TRV3; blue trace), or vehicle (dashed lines) at the indicated time point. The BRET records are the average of three independent experiments. Mean values ± S.E.M. are shown (n = 3). Stim, stimulation.
BRET Assay for Detection of Intracellular Processing of AT1-R.
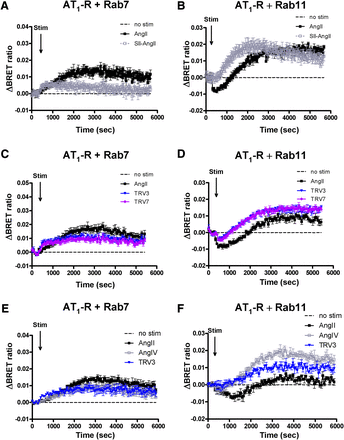
Our results suggested that biased stimulation of AT1-R can lead to altered intracellular processing of the internalized receptor. To investigate this possibility, we performed a BRET experiment to follow AT1-R inside the cell using various markers of endomembranes, such as Rab7 and Rab11, as the markers of degradation and recycling pathways, respectively. As discussed earlier, Rab7-YFP is used to detect the multivesicular body/late endosome pathway and Rab11-YFP is a marker of the late recycling route. As shown in Fig. 7, we detected an elevated BRET ratio with Rab7-YFP following both AngII and biased agonist (SII-AngII, TRV120023, and TRV120027) stimulation. Enhanced initial BRET interactions were detected after stimulation with biased ligands between AT1-R and Rab5 (Fig. 1) and both the Rab7- and Rab11-containing compartments (Fig. 7). The amplitudes of the association of Rab7 and the receptor were in good correlation with the initiated β-arrestin binding upon stimulation with various ligands (Figs. 3 and 7, A, C, and E). In the case of Rab11-YFP and AT1-R–Rluc, AngII stimulus caused an initial decrease in the BRET ratio, but after ∼15 minutes of stimulation, the ratio started to elevate (Fig. 7, B, D, and F; black-filled symbols). The low-affinity agonists caused increased colocalization with Rab11 endosomes after ∼5–10 minutes of the stimulation, which is strikingly different from the AngII-evoked response, where the increased BRET ratio was preceded by a period of ∼15-minute BRET decrease (Fig. 7, B, D, and F). We also analyzed whether the calcium signal of AT1-R is partially responsible for the intracellular processing of the receptor. However, BAPTA-AM pretreatment did not modify the association of AT1-R with Rab4, Rab7, and Rab11 (data not shown), arguing against the role of Ca2+ signal generation in this process.
BRET assay between AT1-R and different Rab proteins upon agonist stimulation (Stim) in HEK293 cells. HEK293 cells were transfected with the plasmids of AT1-R–Rluc and the indicated YFP-fused Rab proteins, and after 24 hours, the cells were exposed to either 100 nM AngII (black-filled symbols), (A and B) 10 μM SII-AngII (gray open symbols), (C and D) 1 μM TRV120023 (TRV3; blue traces), 1 μM TRV120027 (TRV7; purple traces), (E and F) 10 μM AngIV (gray open squares), 1 μM TRV3 (blue traces), or vehicle (dashed lines) at the indicated time points. BRET pairs: (A, C, and E) Rab7-YFP and AT1-R–Rluc; (B, D, and F) Rab11-YFP and AT1-R–Rluc. The BRET records are the average of at least three independent experiments. Mean values ± S.E.M. are shown (n = 3).
Discussion
In the present study, we investigated and analyzed the consequences of biased activation of AT1-R, focusing on β-arrestin binding and intracellular processing of wild-type as well as DRY/AAY mutant AT1-Rs. Agonist activation of most GPCRs, such as AT1-R, initiates phosphorylation of the receptor by G protein–coupled receptor kinases. The phosphorylated carboxyl-terminal tail of the receptor recruits the cytosolic adaptor protein, β-arrestin, and uncouples the receptor from the corresponding heterotrimeric G protein. The receptor bound to β-arrestin is also sorted from the plasma membrane into endocytic vesicles. Receptor internalization can occur by several mechanisms, such as via clathrin-coated vesicles, or by other vesicles, including caveolae (Maxfield and McGraw, 2004). GPCRs, which internalize via the chlathrin-mediated pathway, require agonist binding and subsequent β-arrestin binding to become endocytosed. The adaptor between the phosphorylated receptor and endocytotic machinery is the β-arrestin molecule itself (Ferguson, 2001). AT1-Rs are internalized predominantly via the chlathrin-mediated pathway at physiologic hormone concentrations, but β-arrestin–independent internalization was also reported at higher AngII concentrations (Zhang et al., 1996; Gaborik et al., 2001).
We used a BRET-based approach to investigate the distribution of the receptor in response to ligands that possess biased agonist properties. We used several YFP-labeled fusion constructs and Renilla luciferase–fused receptors as intermolecular probe pairs in BRET measurements. After the addition of AngII, the BRET ratios are increased between AT1-R and Rab5 and Rab7, which are signs of a traveling receptor through endosomes decorated with those proteins (Figs. 1 and 7). Since the DRY/AAY mutation of the receptor or the use of biased agonists (such as SII-AngII, TRV120023, and TRV120027) fail to achieve G protein activation (Gaborik et al., 2003; Wei et al., 2003; Violin et al., 2010), differences were expected in the fate of AT1-R after biased activation. All biased agonists (SII-AngII, TRV120023, and TRV120027) induced colocalization of AT1-R with Rab5 endosomes (indicating internalization of the receptor), which was apparently more robust than the AngII-evoked responses (Fig. 1). The low-affinity unbiased AT1-R agonist AngIV caused a very similar internalization as AngII (Fig. 1C). Our results did not prove the role of either fundamentally different endocytic routes (Supplemental Fig. 3) or a Gq activation–initiated calcium signal (Supplemental Fig. 4) in the background of the dissimilar rate of endocytosis after bias activation of AT1-R. We were also not able to demonstrate correlation between β-arrestin binding and the accelerated internalization of AT1-R after stimulation with biased agonists (Fig. 3). Moreover, it seems that the course of AT1-R–β-arrestin2 recruitment may correlate with the affinity of the ligand since it is known that SII-AngII, TRV120023, and TRV120027 have a significantly lower affinity than AngII (Bonde et al., 2010; Violin et al., 2010), and also the low-affinity unbiased AT1-R agonist AngIV caused a similarly reduced and less stable AT1-R–β-arrestin2 recruitment as the biased agonists (Fig. 3C). We used various concentrations of agonists to check their effects (Supplemental Fig. 5) since locally produced AngII levels in many target tissues are at least one order of magnitude higher than the concentration of circulating AngII (Danser, 2003).
Since both AngII and AngIV initiated PtdIns(4,5)P2 breakdown in our system, whereas the biased agonists did not (Supplemental Fig. 1), we investigated the role of PtdIns(4,5)P2 in the regulation of AT1-R internalization. The pharmacological approach revealed that PI4KIIIα is responsible for the maintenance of the PtdIns(4,5)P2 pool, which is involved in receptor-mediated endocytosis since both 10 μM wortmannin and 10 nM A1 were able to suspend the endocytosis after AngII or AngIV stimulation of AT1-R (Figs. 4 and 5). The inhibitory effect of the inhibition of PtdIns(4,5)P2 resynthesis by the blockade of PI4KIIIα activity on AT1-R endocytosis is consistent with the observation that PtdIns(4,5)P2 depletion by a rapamycin-inducible heterodimerization system also interfered with GPCR internalization (Supplemental Fig. 7) (Toth et al., 2012).
Several studies have investigated the roles of different Rab proteins, such as Rab4, Rab5, Rab7, and Rab11, in AT1-R intracellular processing (Hunyady et al., 2002; Seachrist et al., 2002; Seachrist and Ferguson, 2003; Dale et al., 2004). We extended those studies using biased agonists in living cell experiments. It is important to emphasize in the interpretation of the results of the previous studies and our recent studies that a given intracellular vesicle may contain more than one isoform of Rab proteins (Sonnichsen et al., 2000).
Rab4 shows an overlapping distribution in early endosomes with Rab5 and in recycling endosomes with Rab11. In our studies, the BRET curves of Rab4 and Rab5 with AT1-R were very similar, which suggests that Rab4 is partly located in Rab4/Rab5-positive endosomes (data not shown) in HEK293 cells. As mentioned above, Rab7 is localized to both late endosomal and lysosomal compartments as well (Bucci et al., 2000). Rab7 is important in the regulation of the intracellular processing of GPCRs routing the receptors from early endosomes to late endosomal and lysosomal compartments. It seems that the biased agonists and AngIV-induced receptor trafficking did not prefer the association with Rab7 compartments as much as the AngII-induced mechanism (Fig. 7). Our data raise the possibility that the association with Rab7 is mainly determined by the affinity of the ligands toward the receptor (Fig. 7, A, C, and E). Rab11 is also located in several compartments, such as perinuclear recycling endosomes and the trans-Golgi network, where it influences the slow endosomal recycling and endosome to trans-Golgi network trafficking, respectively. It was demonstrated earlier by confocal (Hunyady et al., 2002) and fluorescence resonance energy transfer microscopy (Li et al., 2008) that Rab4 and Rab11 together coordinate the recycling of AT1-R. During early recycling, the receptor associated mostly with Rab4, and during late-stage recycling, it associated mostly with Rab11. That is in concert with our results, which show that the association of AT1-R with Rab11 is a latter event after stimulation. AngII stimulus caused a drop in the BRET ratio between Rab11-YFP and AT1-R–Rluc, but after ∼15 minutes of stimulation, the ratio started to increase (Fig. 7, B, D, and F; black-filled symbols). This phenomenon could raise the possibility that some fraction of the overexpressed receptor is already located in Rab11 intracellular compartments in HEK293, and after stimulus, these receptors shift to other compartments, which results in the drop in BRET ratio. It is known that Rab11 is a very important player, not just in the regulation of the endocytic recycling compartment, but also in the regulation of the biosynthetic recycling compartment (Saraste and Goud, 2007); moreover, Rab11 regulates exocytosis of vesicles at the plasma membrane (Takahashi et al., 2012). It is possible that AngII stimulation initiates a mechanism, which translocates the preformed or previously endocytosed receptors to the cell surface regulating the responsiveness of the cells.
Interestingly, the association with Rab11 is very dissimilar in the case of stimulation with the other agonists (biased agonists and AngIV), where the association starts at an earlier time point after the stimulation (Fig. 7, B, D, and F). The dissimilarity between AngII and the other ligands suggest that the ligand affinity and strength of arrestin binding can determine the later fate of the stimulated receptor.
Taken together, the wild-type and DRY/AAY mutant AT1-Rs and also the AngII or biased agonist–stimulated receptors differ in their sorting between intracellular compartments. Our data suggest that neither the reduced arrestin binding, fundamentally different internalization routes, nor the calcium signal (Supplemental Figs. 3 and 4), but rather the transient depletion of the plasma membrane PtdIns(4,5)P2 pool, is responsible for the reduced rate of AngII-induced AT1-R endocytosis compared with the biased agonist–induced responses (Figs. 4 and 5; Supplemental Figs. 7 and 8). Even though the main determinant of the endocytic rate is the presence or absence of PLC activation, the later fate of AT1-R within the cells seems mainly dependent on the course of β-arrestin binding to the stimulated receptor. The hormonal responsiveness of tissues and cells is dynamic and determined by the delicate balance between externalization (delivery mechanisms, which transport the receptors from the intracellular compartments to the plasma membrane) and internalization pathways of the receptors. It is very promising that the delicate balance between receptor resensitization/externalization and desensitization/internalization can be modified by biased agonists, which raises the possibility of applying biased ligands in diseases where intracellular receptor processing should be changed.
Acknowledgments
The authors thank Mártonné Schultz for excellent technical assistance.
Authorship Contributions
Participated in research design: Szakadáti, Tóth, Erdélyi, Várnai, Hunyady, A. Balla.
Conducted experiments: Szakadáti, Tóth, Oláh, Erdélyi, A. Balla.
Contributed new reagents or analytic tools: T. Balla.
Performed data analysis: Szakadáti, Tóth, Erdélyi, Várnai, Hunyady, A. Balla.
Wrote or contributed to the writing of the manuscript: Szakadáti, Tóth, Erdélyi, T. Balla, Várnai, Hunyady, A. Balla.
Footnotes
- Received November 11, 2014.
- Accepted March 24, 2015.
This work was supported by the Hungarian Scientific Research Fund OTKA [NK100883 and K105006] and National Development Agency NFU TAMOP [4.2.2-08/1/KMR and 4-2.1/B-09/1/KMR-2010-0001]. A.B. was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. This research was supported in part by the Intramural Research Program of the National Institutes of Health (Section on Molecular Signal Transduction, Program for Developmental Neuroscience, Eunice Kennedy Shriver National Institute of Child Health and Human Development).
↵
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
Abbreviations
- AngII
- angiotensin II
- AngIV
- angiotensin IV
- BRET
- bioluminescence resonance energy transfer
- DN
- dominant-negative
- GFP
- green fluorescent protein
- GPCR
- G protein–coupled receptor
- GRK
- GPCR kinase
- HEK
- human embryonic kidney
- PH
- pleckstrin homology
- PLC
- phospholipase C
- PtdIns(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- Rluc
- Renilla luciferase
- SII-AngII
- [Sar1,Ile4,Ile8]AngII
- Sluc
- super Renilla luciferase
- TRV120023
- Sar-Arg-Val-Tyr-Lys-His-Pro-Ala-OH
- TRV120027
- Sar-Arg-Val-Tyr-Ile-His-Pro-D-Ala-OH
- Wm
- wortmannin
- YFP
- yellow fluorescent protein
- U.S. Government work not protected by U.S. copyright