Abstract
G protein-coupled receptor family C group 6 member A (GPRC6A) is activated by testosterone and modulates prostate cancer progression. Most humans have a GPRC6A variant that contains a recently evolved KGKY insertion/deletion in the third intracellular loop (ICL3) (designated as GPRC6AICL3_KGKY) that replaces the ancestral KGRKLP sequence (GPRC6AICL3_RKLP) present in all other species. In vitro assays purport that human GPRC6AICL3_KGKY is retained intracellularly and lacks function. These findings contrast with ligand-dependent activation and coupling to mammalian target of rapamycin complex 1 (mTORC1) signaling of endogenous human GPRC6AICL3_KGKY in PC-3 cells. To understand these discrepant results, we expressed mouse (mGPRC6AICL3_KGRKLP), human (hGPRC6AICL3_KGKY), and humanized mouse (mGPRC6AICL3_KGKY) GPRC6A into human embryonic kidney 293 cells. Our results demonstrate that mGPRC6AICL3_KGRKLP acts as a classic G protein–coupled receptor, which is expressed at the cell membrane and internalizes in response to ligand activation by testosterone. In contrast, hGPRC6AICL3_KGKY and humanized mouse mGPRC6AICL3_KGKY are retained intracellularly in ligand naive cells, yet exhibit β-arrestin–dependent signaling responses, mitogen-activated protein kinase [i.e., extracellular signal–regulated kinase (ERK)], and p70S6 kinase phosphorylation in response to testosterone, indicating that hGPRC6AICL3_KGKY is functional. Indeed, testosterone stimulates time- and dose-dependent activation of ERK, protein kinase B, and mTORC1 signaling in wild-type PC-3 cells that express endogenous GPRC6AICL3_KGKY. In addition, testosterone stimulates GPRC6A-dependent cell proliferation in wild-type PC-3 cells and inhibits autophagy by activating mTORC1 effectors eukaryotic translation initiation factor 4E binding protein 1 and Unc-51 like autophagy activating kinase 1. Testosterone activation of GPRC6A has the obligate requirement for calcium in the incubation media. In contrast, in GPRC6A-deficient cells, the effect of testosterone to activate downstream signaling is abolished, indicating that human GPRC6A is required for mediating the effects of testosterone on cell proliferation and autophagy.
Introduction
G protein-coupled receptor family C group 6 member A (GPRC6A) is a member of the class-C G protein–coupled receptor (GPCR) family that is widely expressed and activated by multiple ligands, including the peptide osteocalcin (Ocn), cations, basic amino acids, and testosterone (Pi et al., 2016b). The linkage of a Venus flytrap (VFT) motif to the classic GPCR 7-transmembrane domain accounts for the ligand diversity of GPRC6A (Pi et al., 2012, 2015, 2018a). Mouse GPRC6A is proposed to regulate complex endocrine networks and metabolic processes, as demonstrated in in vitro cell culture and in vivo animal models (Ferron et al., 2010; Karsenty and Olson, 2016; Pi et al., 2016a,b). Genetic loss-of-function studies and pharmacological activation of GPRC6A in mice show that GPRC6A regulates insulin secretion and proliferation by β-cells (Pi et al., 2011, 2012; Oury et al., 2013; Wei et al., 2014), glucose uptake and interleukin 6 secretion by skeletal muscle (Mera et al., 2016), testosterone secretion by testicular Leydig cells (De Toni et al., 2014), glucose and fat metabolism by hepatocytes and adipocytes (Otani et al., 2015), and prostate cancer progression (Pi and Quarles, 2012). GPRC6A in these tissues senses Ocn released from bone to create endocrine networks regulating energy homeostasis and sexual reproduction (Pi et al., 2016b). GPRC6A also mediates the rapid, nongenomic effects of testosterone in peripheral tissues, including Leydig cells, β-cells, skeletal muscle, and skin keratinocytes (Pi et al., 2010, 2015; Ko et al., 2014).
A KGKY polymorphism in the third intracellular loop (ICL3) of GPRC6A evolved in most humans (GPRC6AICL3_KGKY) (Jørgensen et al., 2017) to replace the ancestral KGRKLP (GPRC6AICL3_KGRKLP, rs386705086) sequence present in all animal species. Human embryonic kidney (HEK) 293 cells transfected with mouse GPRC6AICL_KGRKLP show that this ancestral variant localizes to the cell surface membrane, and imparts signaling responses to Ocn, testosterone, L-Arginine, and cations (Pi et al., 2005, 2010, 2012, 2015, 2016a, 2018a; Oury et al., 2013; Mera et al., 2016; Ye et al., 2017). In contrast, human GPRC6AICL3_KGKY is proposed to be a loss-of-function polymorphism that is not associated with any human diseases (Jørgensen et al., 2017). Studies of a human cDNA transfected into HEK-293 cells found that the GPRC6AICL3_KGKY variant is retained intracellularly and lacks responsiveness to Ocn and testosterone (Jacobsen et al., 2017; Jørgensen et al., 2017). If this scenario is correct, a functional GPRC6A in humans would be limited to the 20% of Caucasians and 40% of people of African decent that express the ancestral KGRKLP polymorphism (Pi et al., 2016b).
In the current study, we determined the distribution of transfected mouse, human GPRC6A, and humanized mouse GPRC6AICL3_KGKY constructs in naive and testosterone-stimulated HEK-293 cells. We also examined the function effects of GPRC6A ligands in wild-type (WT) PC-3 cells expressing endogenous GPRC6AICL3_KGKY and in cells after ablation of GPRC6A. We found that GPRC6AICL3_KGRKLP is cell surface expressed, imparts second messenger signaling, and undergoes internalization in response to GPRC6A agonists, consistent with a classic GPCR. In contrast, we confirmed that GPRC6AICL3_KGKY is retained intracellularly, but leads to mitogen-activated protein kinase [i.e., extracellular signal–regulated kinase (ERK)] and mTORC1 activation in response to ligands. Thus, the GPRC6AICL3_KGKY polymorphism is not a loss-of-function polymorphism but may have evolved to link to nutrient sensing to ERK and mTORC1 pathways after internalization to endosomes.
Materials and Methods
Establishment of CRISPR/Cas9-Mediated GPRC6A Knockout Cell Clones.
In the present study, one GPRC6A knockout (KO) single clone B12 was selected from our previously established CRISPR/Cas9-edited GPRC6A PC-3 cell line (Ye et al., 2017). We obtained a total of 11 single clones using a serial dilution method, in which B12 contained the 2nt insert (the sequence was confirmed by T-A cloning sequencing) and caused frameshift mutation and loss of function in response to ligand stimulation (for details, see Supplemental Figs. 1 and 2).
Cell Culture and Treatment.
Cells were cultured in RPMI 1640 media and cell proliferation assays as previously described (Ye et al., 2017). Briefly, 4 × 103 cells were seeded in the each well of a 96-well plate, with or without 50 nM testosterone, and the 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide dye production (MTT) method was used to measure for cell proliferation (Cayman Chemical). For treatment, cells were placed in Hanks’ balanced salt solution (Thermo Fisher Scientific), supplemented with 0.5 mM Ca2+ if not otherwise stated, for 2 to 3 hours before stimulation. For autophagy assay, we used GPRC6A KO (B12) and GPRC6A WT control cells (with Cas9 expression and without short guide RNA expression) to examine autophagy marker light chain 3 (LC3) B level and phosphorylation of Unc-51 like autophagy activating kinase 1. Cell autophagy is induced by 5-hour starvation in Hanks’ balanced salt solution with 50 μM chloroquine (Lee et al., 2007). In addition, both GPRC6A KO cells and control cells were transfected with RFP-LC3 expression plasmids and established stable cell lines, and the cells were further used for LC3 punctate measurement. Images were taken using a Nikon Ti inverted fluorescence microscope.
Reverse Transcription Polymerase Chain Reaction and Real-Time Polymerase Chain Reaction.
The reverse transcription polymerase chain reaction assay was performed as in previous studies (Pi et al., 2010). The primers used for reverse transcription polymerase chain reaction and real-time polymerase chain reaction are listed in Supplemental Table 1.
Western Blot.
Western blots were performed as previously described (Ye et al., 2017). Briefly, after 48-hour culture, quiescence was achieved in subconfluent cultures by removing the medium and washing with Hanks’ balanced salt solution to remove residual serum, followed by incubation for an additional 4 hours in serum-free medium (Dulbecco’s modified Eagle’s medium/F-12 medium containing 0.1% bovine serum albumin and about 1 mM calcium; Thermo Fisher Scientific). After agonist treatment at the specified concentrations and duration, cells were washed twice with ice-cold PBS and scraped into 250 μl of LDS sample buffer (Thermo Fisher Scientific) with 0.02 ml per tablet of protease inhibitor (Thermo Fisher Scientific). Equal amounts of lysates were subjected to 10% SDS-PAGE, and target protein levels were determined by immunoblotting using antibodies. The antibodies used for these analyses are listed in Supplemental Table 2. Western blot intensity was analyzed by ImageJ software (https://imagej.nih.gov/ij/).
Confocal Microscopy Procedures.
Cells stably expressing myc-tagged mouse WT GPRC6A (mGPRC6AICL3_KGRKLP) or myc-tagged mouse GPRC6AICL3_KGKY were cultured in Dulbecco’s modified Eagle’s medium + 10% FBS supplemented with 200 µg/ml of G418. Cells plated on laminin-coated glass coverslips were pre-exposed to buffer or to 30 µM Dyngo-4a for 30 minutes and then to 1 µM testosterone for an additional 30 minutes (Li et al., 2013). Thereafter, slides were fixed in PBS containing 4% paraformaldehyde and 4% sucrose (pH 7.4) for 10 minutes at room temperature (Bahouth and Nooh, 2017). To permeabilize the cells, fixed slides were incubated in HEPES-buffered saline solution containing 0.2% Triton X-100 for 10 minutes at 4°C, followed by three washes in ice-cold PBS (Li et al., 2013). At this stage, coded slides were blocked with 5% bovine serum albumin in PBS for 30 minutes at room temperature. Cells expressing mGPRC6AICL3_KGRKLP or mGPRC6AICL3_KGKY were incubated with 1:250 dilution of monoclonal anti-9E10 c-Myc antibody in PBS or 5% FBS in PBS for permeabilized cells for 1 hour at room temperature. Similarly, cells expressing human GPRC6A (hGPRC6AICL3_KGKY) were incubated with 1:250 dilution of rabbit anti-GPRC6A antibody in PBS for nonpermeabilized cells and 5% FBS in PBS for permeabilized cells. Cells expressing mGPRC6AICL3_KGRKLP or mGPRC6AICL3_KGKY were incubated with 1:250 dilution of goat anti-mouse IgG conjugated to Alexa Fluor 555, while cells expressing hGPR6CA were incubated in 1:500 dilution of CF-633 conjugated to Donkey anti-rabbit IgG for 1 hour at room temperature. These antibodies were diluted in PBS for nonpermeabilized cells or PBS containing 5% FBS for permeabilized cells. The slides were washed three times in PBS and then mounted on microscopic glass slides for confocal microscopy. Confocal fluorescence microscopy was performed at room temperature on coded slides and optical section thicknesses of 1.0 mm images were acquired by an Olympus FV1000 confocal microscope equipped with a 40 or 60 Å oil immersion (numerical aperture 1.30) objective lens, using FV10-ASW 3.1 acquisition software (Olympus, Center Valley, PA) (Bahouth and Nooh, 2017). TIFF files of each image were exported and analyzed for pixel intensity and distribution by the ImageJ software. Each cell was partitioned by a line, every point of which was at a distance of 300 nm from the outer periphery of the cell, using the ImageJ software. This line formed the outer limit of the area used to index pixel intensity of internal GPRC6A in a given cell, while the area formed between the peripheral cell membrane and this line was used to index the distribution of pixels associated with cell surface GPRC6A (Gardner et al., 2007; Li et al., 2013; Bahouth and Nooh, 2017).
Statistics.
Data were derived from image analysis that determined specific total pixels and pixels outside versus inside the 300-nm partition that was drawn around the inner circumference of cardiomyocytes. The ratio of pixels residing outside the 300-nm partition to that of the percentage of total pixels was calculated for each image. The average ± S.E. of percentile pixel ratios from three separate experiments derived from 10 images/experiment (n = 30 images) is presented. Statistical comparison between two groups was performed by unpaired t tests and for multiple groups by ANOVA (Tsvetanova and von Zastrow, 2014), followed by Bonferroni’s test with a single pooled variance test in which the family-wise error rate was set at 0.05, using GraphPad Prism 7 software (GraphPad Software Inc.). Regarding the data obtained from western blot, cell proliferation, and autophagy assay, the statistical significance of differences between the two groups was calculated by using Student’s t test. Other statistical analyses performed were Dunnett’s or Tukey-Kramer’s tests, as post-hoc tests following ANOVA.
Results
Dose and Time Dependence of Testosterone-Mediated Activation of ERK/Phosphoinositide 3-Kinase/Protein Kinase B/mTORC1 Signaling in PC-3 Cells that Express the Endogenous GPRC6AICL3-KGKY Polymorphism.
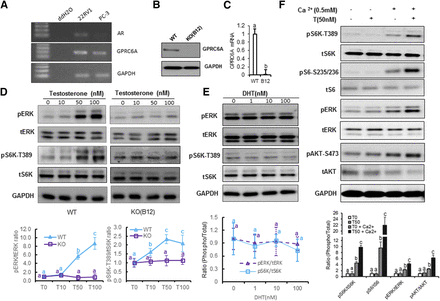
PC-3 cells endogenously express human GPRC6AICL3_KGKY but not androgen receptor (AR) transcripts (Ye et al., 2017), making them a model to study the nongenomic, AR-independent effects of testosterone (Fig. 1A). The human prostate cancer cell, 22RV1, highly expressed GPRC6A and AR (Pi and Quarles, 2012); therefore, we used 22RV1 cells as the positive control. To create a PC-3 cell line with ablated GPRC6A, we used the CRISPR/Cas9 system to delete the hGPRC6A gene (Supplemental Figs. 1 and 2). We selected a PC-3 cell clone, termed B12 (PC-3/GPRC6AKO-B12), which lacked the mRNA and protein of hGPRC6A (Fig. 1, B and C) and used it along with WT PC-3 cells to determine if ablation of hGPRC6A was associated with loss of downstream signaling by testosterone.
GPRC6A directly mediated in testosterone-induced mTORC1 activation. (A) Reverse transcription polymerase chain reaction (PCR) analysis of AR and GPRC6A expression in PC-3 cells. 22Rv1 was employed as a positive control for the AR and GPRC6A expression human prostate cancer cell line. (B) Establishment of GPRC6A KO (B12) cell line by the CRISPR/Cas9 system. Western blot analysis of GPRC6A protein level in WT PC-3 cells (with Cas9 expression but no short guide RNA insert) and GPRC6A KO (B12) cells. (C) Real-time PCR of GPRC6A expression in WT PC-3 or KO PC-3 cells. Data are presented as mean ± S.D. Each independent experiment was performed and replicated six times (n = 3). Different letters in the superscripts above the data points indicate significant differences between groups. Values sharing the same superscript letters are not significantly different from each other, and values with different superscript letters indicate significant differences between groups (P < 0.05, Student’s t test.) (D) Knockout of GPRC6A abolished testosterone-induced mTORC1 activation. PC-3 WT cells and GPRC6A KO (B12) cells were treated with different concentrations of testosterone. Cells were incubated in Hanks’ balanced salt solution (HBSS) buffer for 2 hours before 20-minute testosterone stimulation. Data are presented as mean ± S.D. Each independent experiment was performed in biologic triplicates (N = 3). Statistical differences between groups are indicated by superscript letters (P < 0.05, two-way ANOVA with Tukey’s multiple comparisons test), as described for (C). (E) PC-3 cells were incubated in HBSS buffer for 2 hours before 20-minute treatment with dihydrotestosterone (DHT) at different concentrations. No activation is seen by DHT treatment. Statistical differences between groups are indicated by superscript letters, as described in (D). (F) Ca2+ is essential for the activation of mTORC1 and ERK and Akt phosphorylation. PC-3 cells were incubated in HBSS buffer with or without 0.5 mM Ca2+ for 2 hours before 20-minute testosterone stimulation. PC-3 cells were stimulated with HBSS buffer in the presence or absence of 0.5 mM Ca2+. Statistical differences between groups are indicated by superscript letters, as described in (D).
We found that the testosterone dose dependently activated ERK and p70S6 kinase (S6K) phosphorylation in WT human PC-3 cells expressing GPRC6AICL3_KGKY (Fig. 1D, left panel), and this response was lost in PC-3/GPRC6AKO-B12 cells (Fig. 1D, right panel), indicating that human GPRC6A was required for testosterone-mediated signaling responses in PC-3 cells. Next, we tested the more specific AR ligand dihydrotestosterone that is derived from 5-alpha-reductase conversion of testosterone and in previous studies has been shown not to activate GPRC6A (Pi et al., 2010). Dihydrotestosterone failed to induce phosphorylation of ERK or S6K in PC-3 cells at concentrations ranging from 1 to 100 nM (Fig. 1E), consistent with the notion that PC-3 cells lack the AR and in agreement with previous results showing that testosterone but not dihydrotestosterone activated downstream signaling by the hGPRC6A (Pi et al., 2015).
Because the VFT and seven-transmembrane structures of GPRC6A predict activation by cations and allosteric modulators, we reexamined if Ca2+ is required for testosterone-mediated activation of hGPRC6A (Wellendorph and Bräuner-Osborne, 2009; Pi et al., 2010; Oury et al., 2013;White et al., 2013). For these studies, PC-3 cells were incubated in a buffer with or without 0.5 mM Ca2+ and then exposed to testosterone. We found that testosterone failed to induce phosphorylation of ERK, protein kinase B (AKT), S6K, or ribosomal protein S6 (S6) signaling in the buffer without Ca2+ (Fig. 1F). Ca2+ alone weakly stimulated phosphorylation of these downstream signals, but the effects of testosterone on Ca2+ were additive, leading to robust induction of phosphorylation, as previously reported (Pi et al., 2010).
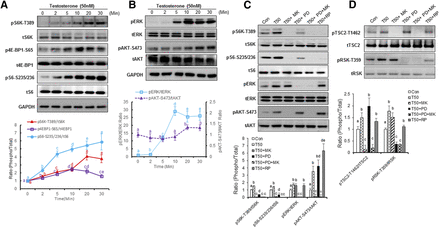
The time course of testosterone-mediated activation of endogenous hGPRC6A was rapid in onset. We observed increased phosphorylation of both ERK and AKT within 2 minutes with maximal activation by 10 minutes that was sustained throughout the 30-minute observation period (Fig. 2A).
Testosterone regulated mTORC1 signaling via ERK and Akt signaling. (A) Time dependence of testosterone-activated mTORC1 signaling. PC-3 cells were assessed at different times after addition of testosterone at a concentration of 50 nM. Data are presented as mean ± S.D. Each independent experiment was performed in biologic triplicates (n = 3). Different letters in the superscripts above the data points indicate significant differences between groups. Values sharing the same superscript letters are not significantly different from each other, and values with different superscript letters indicate significant differences between groups (P < 0.05, two-way ANOVA with Tukey’s multiple comparisons test). (B) Time-dependent testosterone activation of ERK and AKT phosphorylation. PC-3 cells were incubated in Hanks’ balanced salt solution (HBSS) buffer for 2 hours before testosterone (50 nM) stimulation. ERK and AKT activation was analyzed by western blot. Statistical differences between groups are indicated by superscript letters, as described in (A). (C and D) Testosterone-induced mTORC1 signaling activation is inhibited by mTOR, AKT, or ERK inhibitors. PC-3 cells in HBSS were treated with MK2206 [(MK), 1 mM] and/or ERK inhibitor 2′-Amino-3′-methoxyflavone [PD98059(PD), 10 μM], rapamycin [(RP), 100 nM] for 20 minutes. PC-3 cells were incubated in HBSS buffer for 2 hours before 20-minute testosterone stimulation. Statistical differences between groups are indicated by superscript letters, as described in (A).
ERK and AKT are upstream of mTORC1 (Mendoza et al., 2011), an evolutionarily conserved nutrient-sensing pathway (Efeyan et al., 2012; Ben-Sahra and Manning, 2017). Phosphorylations of S6K at Thr389, S6 at Ser235/236, and eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) at Ser65 are characteristic downstream markers for activation of the mTORC1 pathway (Mendoza et al., 2011). Testosterone at concentrations of 50 nM time dependently increased the phosphorylation of pS6K-Thr389, pS6-Ser235/236, and p4E-BP1-Ser65, and their phosphorylation was activated as early as 2 minutes (Fig. 2B).
To further confirm that ERK and AKT mediated testosterone-induced mTORC1 activation, PC-3 cells were treated with testosterone in the presence of 2′-Amino-3′-methoxyflavone (PD98059,ERK inhibitor), and/or 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one [(MK2206), an AKT inhibitor]. Testosterone-induced mTORC1 activation was partially blocked by the ERK inhibitor (2′-Amino-3′-methoxyflavone,PD98059) and completely inhibited by the AKT inhibitor (MK2206) (Fig. 2C). Treatment with the potent AKT inhibitor MK2206 completely inhibited AKT-S473 phosphorylation, but not ERK phosphorylation. In addition, MK2206 inhibited the phosphorylation of S6 at pS6K-T389 and pS6-S235/236, which is a downstream target of mTORC1 activation (Fig. 2C). 2′-Amino-3′-methoxyflavone (PD98059) completely blocked ERK phosphorylation, but had no effect on AKT phosphorylation, and partially inhibited S6 phosphorylation (Fig. 2C). Rapamycin, a mammalian target of rapamycin (mTOR) inhibitor, blocked testosterone-induced S6 phosphorylation in PC-3 cells without affecting ERK or AKT phosphorylation (Fig. 2, C and D).
To define pathways between ERK and AKT activation of mTORC1, we examined the effects of testosterone on phosphorylation of tuberous sclerosis complex 2 and mitogen-activated protein kinase–activated protein 90 kDa ribosomal S6 kinase(RSK). Testosterone significantly stimulated ribosomal S6 kinase phosphorylation in PC-3 cells, and this response was completely blocked by the ERK inhibitor, partially inhibited by rapamycin, and not affected by the AKT inhibitor. Conversely, testosterone also induced tuberous sclerosis complex 2 phosphorylation at Thr1462, and this response was blocked by the AKT inhibitor but not the ERK inhibitor (Fig. 2D). These findings suggest that ERK/90 kDa ribosomal S6 kinase and AKT are two independent pathways involved in testosterone-induced mTORC1 activation in PC-3 cells.
GPRC6A Regulates Cell Proliferation and Autophagy through mTORC1 Signaling.
Since mTORC1 is a master regulator of cell growth, metabolism, and survival (Laplante and Sabatini, 2009), we determined the role of mTORC1 in mediating the function of hGPRC6A in PC-3 cells.
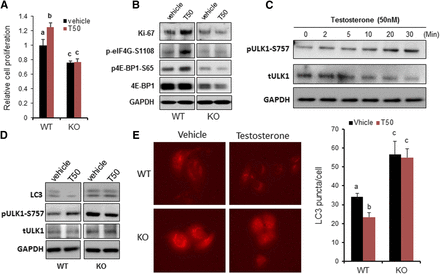
First, we examined the effects of testosterone on cell survival and proliferation in the WT PC-3/GPRC6AICL3-KGKY and GPRC6A KO PC-3/GPRC6AKO-B12 cell lines. These cells were seeded at the same density and incubated for 2 days in the presence or absence of testosterone (Cvicek et al., 2016). We consistently counted fewer PC-3/GPRC6AKO-B12 cells compared with WT PC-3 hGPRC6A cells leading to impaired cell proliferation and/or decreased survival. We speculate that these effects were mediated by hGPRC6A, because testosterone significantly increased the number of cells in WT PC-3 cells expressing the endogenous GPRC6AICL3-KGKY variant but not in PC-3/GPRC6AKO-B12 cells (Fig. 3A). Testosterone also induced expression of the cell proliferation marker Ki-67 (Fig. 3B) and phosphorylation of 4E-BP1 and eIF4G, two effectors of mTORC1 signaling that are responsible for regulating the proliferation of PC-3/GPRC6AICL3-KGKY cells. However, in hGPRC6A-null PC-3 (PC-3/GPRC6AKO-B12) cells, the effect of testosterone on these parameters was completely abolished (Fig. 3B).
GPRC6A mediated in testosterone-induced cell proliferation and autophagy. (A) Cell proliferation of PC-3 WT cells and KO (B12) cells using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. On day 0, 3 × 103 per well of cells were seeded in 96-well plates. Cell viability was measured on day 2. Cells were cultured in complete RPMI 1640 media with or without 50 nM of testosterone (T50). Data are mean ± S.D. (n = 8). Different letters in superscripts above the data points indicate significant differences between groups. Values sharing the same superscript letters are not significantly different from each other, and values with different superscript letters indicate significant differences between groups (P < 0.05, two-way ANOVA with Tukey’s multiple comparisons test). (B) Western analysis of cell proliferation marker Ki67 expression and mTORC1 activation. Cells were cultured in complete RPMI 1640 media with or without T50 for 48 hours. (C) Time course of testosterone on Unc-51 like autophagy activating kinase 1 (ULK1) activation. Phosphorylation of ULK1 at Ser757 was measured using western blot. PC-3 WT cells were incubated in Hanks’ balanced salt solution (HBSS) buffer for 2 hours before 20-minute T50 stimulation. (D) Western blot analysis of LC3B level and ULK1 activation. Cell autophagy was induced by 5-hour starvation in HBSS with 50 μM chloroquine (Lee et al., 2007). (E) Representative LC3 punctate images of PC-3 WT cells and PC-3 GPRC6A-KO (B12) cells stably expressing LC3-RFP. Autophagy is induced by 5-hour starvation in HBSS buffer with 50 μM chloroquine (Lee et al., 2007). Scale bar, 20 μm. LC3 punctate was quantitated by ImageJ software. Data are mean ± S.D. of LC3 punctate/cell. Statistical differences between groups are indicated by superscript letters, as described in (A).
Next, we examined if human GPRC6A was involved in regulating autophagy in PC-3 cells because mTORC1 is a major inhibitor of autophagy, and testosterone has been shown to inhibit autophagy (Tomaszewski et al., 2015). Since mTORC1 suppressed autophagy via phosphorylating Unc-51–like autophagy activating kinase-1 ULK1 at Ser757, we examined if testosterone would further increase the phosphorylation of ULK-1 on Ser757. Testosterone induced a time-dependent increase in the phosphorylation of ULK-Ser757, achieving a maximal response by 30 minutes (Fig. 3C). Testosterone-mediated increase in the phosphorylation of ULK-Ser757 was observed in WT and PC-3/GPRC6AICL3-KGKY cells, but not in GPRC6A null PC-3/GPRC6AKO-B12 cells (Fig. 3D).
The autophagy-related protein LC3 is a widely used marker of autophagosomes, where the lapidated form of LC3-II indexes autophagic activity (Yoshii and Mizushima, 2017). Western blot analysis showed that phosphorylation of lipidated LC3-II was decreased in PC-3/GPRC6AICL3-KGKY cells after testosterone stimulation, whereas basal phosphorylation was increased in vehicle-treated PC-3/GPRC6AKO-B12 cells that exhibited no response to testosterone (Fig. 3D). PC-3/GPRC6AKO-B12 cells also exhibited increased formation of LC3 autophagosomes, while testosterone reduced LC3-II expression in PC-3/GPRC6AICL3-KGKYcells, an effect blocked by deletion of GPRC6A blocked in PC-3/GPRC6AKO-B12 (Fig. 3E).
Collectively, these results unequivocally establish the function of endogenous human GPRC6AICL3-KGKY, and contrast with the reported lack of function of a transfected hGPRC6A construct, which was found to be retained intracellularly (Jørgensen et al., 2017). We propose that hGPRC6A is an example of a GPCR that exhibits ligand-dependent signaling after endocytosis (Thomsen et al., 2016; Cahill et al., 2017).
Cellular Localization and Ligand-Dependent Activation of the GPRC6A KGKY and RKLP Polymorphisms.
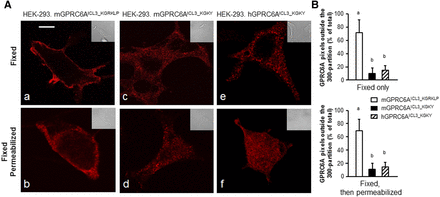
To examine the impact of the KGKY and KGRKLP motifs on ligand-dependent endocytosis, we transfected WT human GPRC6A (GPRC6AICL3_KGKY), Myc-tagged WT mouse GPR6CA (GPRC6AICL3_KGRKLP), and Myc-tagged humanized mouse GPRC6A (Myc-mGPRC6AICL3_KGKY), which were created by replacing KGRKLP with KGKY in the ICL3 of mGPRC6A into HEK-293 cells. The cells were fixed and then either used as such or permeabilized as described in Materials and Methods. The cells were then incubated with primary monoclonal (9E10) anti-Myc or rabbit anti-human GPR6CA antibodies, followed by secondary fluorescent anti-mouse or anti-rabbit IgG. Confocal fluorescence microscopy indicated that in HEK-293 cells, ancestral mGPRC6A (GPRC6AICL3_KGRKLP) pixels were predominately located (70% ± 14%) in the cell surface membrane of intact or permeabilized HEK-293 cells (Fig. 4A, images a and b, respectively). In contrast, in HEK-293 cells, humanized mouse GPRC6A (mGPRC6AICL3_KGKY) (Fig. 4A, images c and d), and human GPRC6A (Fig. 4A, images e and f) were mostly intracellular. These receptors were distributed by ∼12% and 17% on the cell surface and by 88% ± 17% and 83% ± 15% internally (Fig. 4B), respectively (n = 30 images derived from three separate experiments).
Cellular compartmentalization of the various GPRC6A constructs in HEK-293 cells. (A) Distribution of the various GPRC6A constructs in HEK-293 cells. Fluorescence confocal microscopy of cells expressing the indicated GPR6A constructs that were either fixed (images a, c, and e) or fixed and then permeabilized (images b, d, and f). The distribution of pixels was obtained by confocal microscopy and the colors shown are pseudo colors, while the inset represents Nomarski images that were acquired at the same magnification. These images are representative of images derived from three separate experiments with 10 aggregate images per condition. Scale bar, 5 μm. (B) Digitized difference GPRC6A membrane location. Data are mean ± S.D. of GPRC6A pixels outside a 300-nm partition derived from 30 images derived from n = 3 experiments. Different letters in the superscripts above the data points indicate significant differences between groups. Values sharing the same superscript letters are not significantly different from each other, and values with different superscript letters indicate significant differences between groups (P < 0.05, two-way ANOVA with Tukey’s multiple comparisons test).
Consistent with findings in classic GPCR, activation of mGPRC6A with testosterone induced the redistribution of the pixels from the membrane to internal puncta that had a diameter of 300–500 nm, commonly associated with endosomal structures (Fig. 5A, images a and b). In contrast, addition of testosterone, which activates hGPRC6A, did not alter the distribution of internal humanized mGPRC6A (GPRC6AICL3_KGKY) (Supplemental Fig. 3).
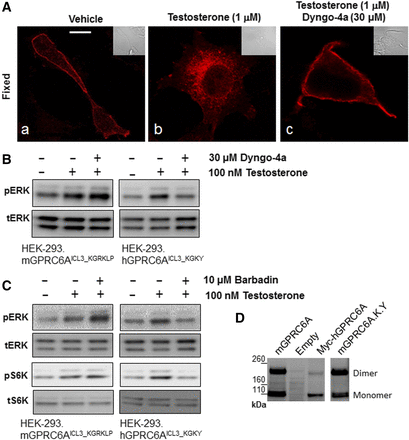
Comparison of intracellular function of mouse and human GPRC6A in HEK-293 cells. (A) Dyngo-4a inhibited GPRC6A ligand; testosterone induced receptor internalization. The slides were pretreated with diluent or 30 µM Dyngo-4a for 30 minutes and then exposed to 1 µM testosterone for 30 minutes and fixed (images b and c, respectively). Scale bar, 5 μm. (B and C) Comparison of Dyngo-4a and Barbadin regulated GPRC6A-mediated ligand; testosterone induced ERK and S6K phosphorylation in HEK-293 cells stably transfected with WT mouse and human GPRC6A (mGPRC6AICL3_KGRKLP and hGPRC6AICL3_KGKY) cDNAs. Cells were preincubated with vehicle, Dyngo-4a (30 μM (Ab120689; Abcam), or Barbadin (10 μM) (Axon2774; Axon Medchem) for 20 minutes after testosterone treatment. (D) Comparison of dimerization of the various GPRC6As in HEK-293 cells, WT mouse GPRC6A (mGPRC6AICL3_KGRKLP) vs. humanized mouse GPRC6A (mGPRC6AICL3_KGRKLP) or human GPRC6A (hGPRC6AICL3_KGKY), analyzed by western blots: 16 μg of each plasmid cDNA was transfected into HEK-293 cells (1.5 × 106 cells/100 mm dish) and 100 μg of each total protein sample was loaded on 3%–8% SDS-PAGE gels. Anti-Myc antibody was used in the western blot analysis. These images are representative of images derived from three separate experiments.
Translocation of mGPRC6A in response to testosterone was inhibited in cells pretreated with the potent dynamin inhibitor Dyngo-4a (Robertson et al., 2014) (Fig. 5A, image c). To confirm Dyngo-4a blocks mGPRC6A-mediated testosterone-induced translocation and function, we compared the functions of mGPRC6A and hGPRC6A in testosterone-induced intracellular ERK phosphorylation. In contrast, we found that Dyngo-4a enhanced testosterone-induced ERK phosphorylation in mGPRC6A, but inhibited testosterone-induced ERK activity in hGPRC6A-transfected HEK-293 cells (Fig. 5B).
β-Arrestins are multifunctional endocytic adaptors and signal transducers (Lefkowitz and Shenoy, 2005). Next, we studied if β-arrestins are involved in GPRC6A endocytosis using the β-arrestin inhibitor, Barbadin (Beautrait et al., 2017). Like Dyngo-4a, Barbadin (10 μM) enhanced testosterone-induced ERK phosphorylation in mGPRC6A-transfected HEK-293 cells, but inhibited testosterone-induced ERK phosphorylation in hGPRC6A-transfected HEK-293 cells (Fig. 5C, upper panel). Interestingly, Barbadin also increased activation of S6K in mGPRC6A, but decreased S6K phosphorylation in hGPRC6A-transfected HEK-293 cells (Fig. 5C, middle panel).
These findings are consistent with prior studies showing desensitization of mouse GPRC6A signaling through a β-arrestin–dependent classic rapid recycling mechanism, and the findings of Jørgensen et al. (2017) showing that the KY insertion/deletion in GPRC6A is mostly retained intracellularly and recycles through a β-arrestin–dependent slow recycling mechanism (Lefkowitz and Shenoy, 2005; Pi et al., 2005).
Finally, we compared the effect of KGKY on western blot migration of epitope-tagged mGPRC6A, humanized mouse GPRC6A, and human GPRC6A using Myc antibody. The estimated molecular weight of the GPRC6A monomer is 110 kDa, and GPRC6A is known to form a dimer with molecular weight of ∼220 kDa (Nørskov-Lauritsen et al., 2015; Ye et al., 2017). We found that the human GPRC6A dimerized to a lesser extent compared with the mouse GPRC6A, as evidenced by the altered distribution of GPRC6A monomers and dimers in HEK-293 cells transfected with these constructs (Fig. 5D). Interestingly, both WT mouse mGPRC6AICL3-KGRKLP and humanized mGPRC6AICL3-KGKY showed equal amounts of 110 and 220 kDa protein bands, corresponding to monomer and dimer migration (1.23 or 1.18, dimer/monomer ratio, respectively), whereas WT human GPRC6AICL3-KGKY showed a greater ratio of monomer to dimer (0.32 dimer/monomer ratio), suggesting that the polymorphisms in the ICL3 are not controlling dimerization (Fig. 5D).
Discussion
A newly evolved human GPRC6A ortholog with the ICL3_KGKY polymorphism is reported to be retained intracellularly and to lack ligand-dependent activation (Jørgensen et al., 2017). These negative findings, along with failure to identify any association between GPRC6A mutations and human diseases in a Danish cohort have raised questions about the biologic relevance of GPRC6A in humans. However, our data show that the human GPRC6AICL3_KGKY variant, although predominantly intracellular, can be detected in the plasma membrane, and more importantly is functional as evidenced by its activation by testosterone and other agonists, as previously reported (Pi et al., 2016a, 2018a,b).
The current studies extend our understanding of the important difference between GPRC6A membrane locations imparted by replacement of the ancestral RKLP sequence with the KGKY insertion/deletion in the ICL3. We show that mouse GPRC6A with the ancestral RKLP polymorphism in the ICL3 acts like a classic GPCR in that mGPRC6A is predominately expressed in the cell surface membrane and undergoes ligand-dependent internalization, perhaps via clathrin-coated pit-mediated endocytosis. On the other hand, human GPRC6AICL3_KGKY is present at low levels in the cell membrane (∼20%) and is predominately located (∼80%) in the endosome-like intracellular punctuate structures, consistent with the observation that human GPRC6A is largely retained intracellularly and undergoes slow recycling from the endosome (Jacobsen et al., 2017). The molecular mechanism whereby replacing the KGRKLP sequence with KGKY leads to intracellular retention needs further study. Since the KGRKLP motif is present in family C members that undergo desensitization and recycling to the plasma membrane, it is tempting to speculate that the KGRKLP sequence represents a membrane retention motif, and the replacement with the KGKY motif leads to loss of binding to essential proteins, leading to GPCR cycling to endosomes (Lefkowitz and Shenoy, 2005).
Regardless, and most importantly, in contrast to the purported lack of ligand-dependent activation of a transfected human GPRC6A (Jørgensen et al., 2017), we found that endogenous human GPRC6A in PC-3 cells responds to the agonist testosterone by activating ERK and phosphoinositide 3-kinase/AKT signaling pathways. More importantly, ligand activation of GPRC6A is linked to downstream mTOR activation. The cell surface expressed human GPRC6A may likely account for our previous observations showing that coexpression of β-arrestin 1 and 2, C3 toxin, or pertussis toxin pretreatment blocked the effects of cations to activate transfected human GPRC6A (Pi et al., 2005). The endosomal signaling by internalized GPRC6A is similar to the evolutionarily conserved pathway in which L-arginine activates mTORC1 signaling in lysosomes from yeast to mammals (Kapahi et al., 2010; Efeyan et al., 2012; Saxton and Sabatini, 2017). Similar to the lysosomal nutrient sensing pathway, testosterone activation of GPRC6A in the endosomes may also result in the inhibition of autophagy and induction of anabolic cellular responses through activation of S6 kinase and inhibition of repressor polypeptide 4E-BP1 and ULK1.
Our current findings are consistent with prior observations that humanizing the mouse GPRC6A by replacing the KGRKLP sequence with KGKY prolonged mTOR signaling, suggesting the internalized KGKY polymorphism is a gain-of-function variant (Ye et al., 2017), but not consistent with the reported agonist-independent internalization and slow recycling from the endosome of human GPRC6A reported by others (Jacobsen et al., 2017).
The structural basis for sensing of cations and L-arginine resides in the VFT, and testosterone, Ocn, and other ligands are imparted by the VFT and seven-transmembrane domain structure (Pi et al., 2005, 2015, 2016a, 2018a). The inability to show ligand-dependent activation of GPRC6A by other investigators may be due to inclusion of a wash step that depleted essential cofactors necessary for ligand activation of GPRC6A. Consistent with this possibility, we found that testosterone activation of GPRC6A is dependent on media calcium concentration of at least 0.5 mM. Physiologically, GPRC6A is always in the presence of calcium above the activating threshold, whereas the concentrations of testosterone, Ocn, and other ligands may vary to activate GPRC6A. Our studies examine the function of GPRC6A in PC-3 cells that do not express the AR; however, we have shown that GPRC6A has a similar function in 22RV1 cells that express the AR (Pi and Quarles, 2012).
Thus, rather than being a nonfunctional variant, human GPRC6A appears to belong to the growing examples of GPCRs that exhibit endosomal signaling after ligand-mediated internalization (Thomsen et al., 2016; Cahill et al., 2017). In the case of GPRC6AICL3_KGKY the internal signaling is linked to ERK and mTOR, suggesting that the KGKY polymorphism may have evolved to provide a means for a GPCR to activate mTORC1 in endosomes/lysosomes. Additional studies are needed to define the intracellular compartments in which GPRC6A activates mTOR signaling.
Regardless, the potential biologic significance of GPRC6A is shown by studies of mouse GPRC6A, indicating important roles in regulating energy metabolism and prostate cancer progression (Pi et al., 2010, 2011, 2016b; Pi and Quarles, 2012; Oury et al., 2013; Di Nisio et al., 2017). GPRC6A antagonists would inhibit both AKT/mTOR and ERK/mitogen-activated protein kinase signaling, which is an effective method for treating hormone-refractory prostate cancer (Kinkade et al., 2008). If the human GPRC6AICL3_KGKY has similar functions to mouse GPRC6A, it may account for racial disparities in susceptibility to metabolic disorders and prostate cancer (Cho, 2011; Laplante and Sabatini, 2012).
Presently, clinical studies show that levels of the GPRC6A ligand, Ocn, are inversely associated with glycemic status, insulin resistance, and obesity in humans (Pittas et al., 2009; Foresta et al., 2011; Iki et al., 2012). Genome-wide association studies find association with GPRC6A polymorphisms with insulin resistance (Di Nisio et al., 2017) testicular failure (Oury et al., 2013; De Toni et al., 2016), and prostate cancer (Takata et al., 2010; Long et al., 2012). However, a recent study of a Danish cohort failed to identify any association between GPRC6A inactivating mutations and human disease (Jørgensen et al., 2017). Analysis of genome-wide association studies is confounded because the KGKY polymorphism is the referent sequence in humans and the RKLP polymorphism is not detected by current gene chips used to genotype patient cohorts.
In conclusion, the emergence of signaling of endogenous hu GPRC6AICL3_KGKY only in hominids and its preservation during evolution indicates selection pressures that modified the functions of GPRC6A to promote ligand-depedent intracellular signaling and coupling to ERK and mTOR. Further studies are needed to establish the clinical relevance of GPRC6A in human pathophysiology.
Acknowledgments
We acknowledge Dr. Malia B. Potts for gifting the GFP-RFP-LC-3 plasmid.
Authorship Contribution
Participated in research design: Ye, Pi, Quarles.
Conducted experiments: Ye, Pi, Nooh, Bahout.
Performed data analysis: Ye, Pi, Bahout, Quarles.
Wrote or contributed to the writing of the manuscript: Ye, Pi, Bahout, Quarles.
Footnotes
- Received October 24, 2018.
- Accepted March 6, 2019.
Funding was provided by the National Institutes of Health [Grant R01-AR37308] and the American Diabetes Association [Grant 1-13-BS-149-BR (to L.D.Q.)].
The authors declare that they have no conflicts of interest with the contents of this article.
↵
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
Abbreviations
- 4E-BP1
- eukaryotic translation initiation factor 4E binding protein 1
- AKT
- protein kinase B
- AR
- androgen receptor
- ERK
- extracellular signal–regulated kinase
- GAPDH
- glyceraldehyde 3-phosphate
- GPCR
- G protein-coupled receptor
- GPRC6A
- G protein-coupled receptor family C group 6 member A
- HEK
- human embryonic kidney
- ICL3
- third intracellular loop
- KO
- knockout
- LC3
- autophagy marker light chain 3
- MK2206
- 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one
- mTOR
- mammalian target of rapamycin
- mTORC1
- mTOR complex 1
- Ocn
- osteocalcin
- S6
- ribosomal protein S6
- S6K
- p70S6 kinase
- VFT
- Venus flytrap
- WT
- wild type
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics