Abstract
Activation of pregnane X receptor (PXR) and constitutive androstane receptor (CAR) by xenobiotic inducers of cytochromes P450 is part of a pleiotropic response that includes liver hypertrophy, tumor promotion, effects on lipid homeostasis, and energy metabolism. Here, we describe an acute response to CAR and PXR activators that is associated with induction of Insig-1, a protein with antilipogenic properties. We first observed that activation of CAR and PXR in mouse liver results in activation of Insig-1 along with reduced protein levels of the active form of sterol regulatory element binding protein 1 (Srebp-1). Studies in mice deficient in CAR and PXR revealed that the effect on triglycerides involves these two nuclear receptors. Finally, we identified a functional binding site for CAR and PXR in the Insig-1 gene by in vivo, in vitro, and in silico genomic analysis. Our experiments suggest that activation Insig-1 by drugs leads to reduced levels of active Srebp-1 and consequently to reduced target gene expression including the genes responsible for triglyceride synthesis. The reduction nuclear Srebp-1 by drugs is not observed when Insig-1 expression is repressed by small interfering RNA. In addition, observed that Insig-1 is also a target of AMP-activated kinase, the hepatic activity of which is increased by activators of CAR and PXR and is known to cause a reduction of triglycerides. The fact that drugs that serve as CAR or PXR ligands induce Insig-1 might have clinical consequences and explains alterations lipid levels after drug therapy.
Induction of cytochromes P450 (P450s), other drug-metabolizing enzymes, and drug transporters by their own substrates and other chemicals is an adaptive response of the liver to prevent accumulation of toxic xenobiotics and endobiotics. Xenosensors that mediate this response are the nuclear receptors pregnane X receptor (PXR) and constitutive active/androstane receptor (CAR) (for review, see Handschin and Meyer, 2003). PXR and CAR form heterodimers with the retinoid X receptor (RXR) and bind to specific DNA sequences in the regulatory region of target genes. PXR and CAR induce an overlapping set of genes involved in metabolism and transport of drugs (Maglich et al., 2002) but also genes involved in the regulation of steroids, bile acids, eicosanoids, and genes involved in cholesterol and bile acid homeostasis (Staudinger et al., 2001; Huang et al., 2003).
Insig-1 and Insig-2 are proteins of endoplasmic reticulum (ER) membrane and play an important role in the control of triglyceride and cholesterol biosynthesis (Yabe et al., 2002; Yang et al., 2002). The two isoforms bind in a sterol-dependent fashion to another ER membrane protein, sterol regulatory element binding protein (Srebp) cleavage-activating protein, or Scap, a transport protein needed for escort and subsequent activation of Srebp transcription factors (Hua et al., 1996). When Insig proteins are activated by sterols, insulin or other stimuli, they retain the Scap-Srebp complex in the ER membrane, thereby preventing Srebp-dependent target gene expression. Srebps are a group of basic helix-loop-helix transcription factors, which activate an array of genes involved in the synthesis of cholesterol and triglycerides. Whereas Srebp-2 is mainly involved in cholesterol biosynthesis, Srebp-1a and Srebp1c mainly activate genes involved in fatty acid and triglyceride synthesis (Shimano, 2001).
A decrease in hepatic and/or serum lipids, in particular triglycerides, has been observed in rodents after treatment with inducers of xenobiotic metabolism many years ago (Bjondahl, 1978; Hall et al., 1990; Venkatesan et al., 1994). More recently, known inducers of human drug metabolism such as the commonly used antiretroviral drug efavirenz or the barbiturate phenobarbital (PB) also have been shown to inhibit lipogenesis (El Hadri et al., 2004; Kiyosawa et al., 2004). The molecular mechanism of the effect of inducers on triglycerides has not been explained.
In the present study, we show that nuclear receptors CAR and PXR transcriptionally activate Insig-1 by binding to an enhancer sequence of the Insig-1 gene. Our results explain the negative effect of drugs and xenobiotics on hepatic lipids in vivo and show that CAR and PXR not only play a role in the catabolism of various endogenous and exogenous compounds but also directly affect lipogenic pathways by activating Insig-1. Moreover, because Insig-1 has recently been found to be a possible drug target for the treatment of diabetes (Nakagawa et al., 2006), this study contributes to the understanding of the regulation of this gene and possibly to the development of new therapies against dyslipidemia.
Materials and Methods
Animals. C57BL/6 mice were maintained in a 12-h light/dark cycle and had free access to food and drinking water. Nine- to 11-week-old male animals received an i.p. injection of 100 mg/kg PB (Sigma, Buchs, Switzerland), 10 mg/kg 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP; Bayer AG, Wuppertal, Germany), or 40 mg/kg pregnenolone-16α-carbonitrile (PCN; Sigma) in a 5% dimethyl sulfoxide-corn oil solution i.p. or with vehicle 10 h before dissection. Animals were sacrificed by exposure to CO2, blood was collected by heart puncture, and livers were excised and snap-frozen in liquid nitrogen and stored at -80°C until use.
Analysis of Triglycerides and Cholesterol. Fifty to 100 mg of liver was used for each preparation. After weight determination, liver samples were put in an ethanol/ether [3:1 (v/v)] mixture in FastPrep tubes (Lysing matrix D; Qbiogene, Illkirch, France). Livers were homogenized on the FastPrep instrument for 40 s at position 6.5 and evaporated to complete dryness on a SpeedVac evaporator (Thermo Fisher Scientific, Waltham, MA). Samples were redissolved in 1 ml of isopropanol, and tissue remnants were spun down for 5 min at 14,000g. Six hundred microliters of the supernatant was mixed with 400 μl of water, and lipids were determined using the esterase/oxidase kit for cholesterol determination and the l-α-glycerol phosphate oxidase kit for the determination of triglycerides (Roche Diagnostics, Basel, Switzerland).
RT-PCR Analysis. RNA from cells and tissues was isolated using Tri-Reagent (Sigma). One microgram of total RNA was reverse-transcribed with Moloney murine leukemia virus reverse transcriptase (Roche Diagnostics). PCR was performed using the TaqMan PCR Core Reagent Kit (Applied Biosystems, Foster City, CA) and the transcript level quantitated with an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) according to the manufacturer's protocol. In brief, relative transcript levels were determined using the relative quantitation method by measuring the change in threshold cycle between the gene of interest and the internal control glyceraldehyde-3-phosphate dehydrogenase. Primers and fluorescent probes used in these PCRs are listed in Table 1.
Primers and probes used for RT-quantitative polymerase chain reaction
Reporter Gene Assays. Culture and transfection of CV-1 cells with Lipofectamine transfection reagent (Invitrogen, Carlsbad, CA) was performed as previously published (Handschin et al., 2000). Expression vectors encoding mouse PXR and mouse CAR as well as the β-galactosidase vector used for signal normalization have been described previously (Handschin et al., 2002). For construction of hemagglutinin-tagged vp16 fusion proteins, nuclear receptor sequences were amplified from expression plasmids and subcloned into pcDNA3/vp16-hemagglutinin (HA) (a kind gift from Dr. Dieter Kressler, Biozentrum, University of Basel, Switzerland). Reporter vectors were based on PGL3-LUC (Promega, Madison, WI). Genomic DNA from the murine Insig-1 enhancer region was amplified using PCR primers carrying restriction sites suitable for direct subcloning into the reporter vector.
Preparation of Primary Hepatocytes. For the preparation of mouse hepatocytes animals were anesthetized with ketamine/xylazine (Sigma). The portal vein was cannulated and perfused with HEPES-EGTA, pH 7.4, for 5 min and then with collagenase (type 2; Worthington, Lakewood, NJ) for 6 min. The livers were excised, and cells were filtered through a nylon mesh and centrifuged three times at 50g and 4°C for 5 min each. After determination of viability, cells were plated at a density of 400,000 cells/well (12-well plate) and were allowed to attach for 2 h in Williams' E medium without phenol red (Invitrogen), 10% fetal calf serum, 4 μg/ml insulin, 200 μM glutamine, and 1% penicillin/streptomycin (50 IU/ml) on collagen-coated dishes. Induction experiments were performed in the same medium without fetal calf serum and with reduced insulin (2 μg/ml) but with the addition of 1 μM hydrocortisone.
Primary human hepatocytes in suspension were allowed to attach on collagen-coated plates in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (50 IU/ml), and 1 μM dexamethasone overnight before the start of the experiments. For induction, cells were cultured in Dulbecco's modified Eagle's medium without serum but supplemented with insulin-transferrin-selenium mixture (Sigma) and 1 μM hydrocortisone.
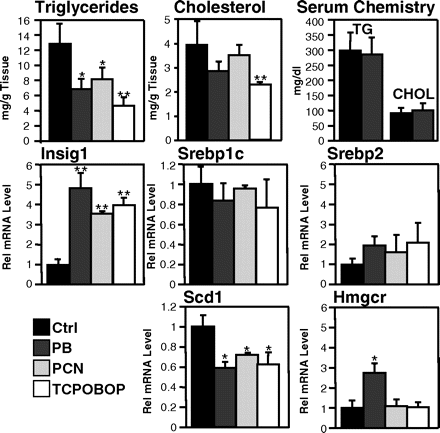
Lipid and gene expression changes after drug application in mouse liver. PB, TCPOBOP and PCN were i.p. injected in mice and livers or serum samples analyzed after 10 h. Top, triglyceride (TG) and total cholesterol (CHOL) analysis in liver homogenates and blood samples. Middle and bottom, RT-PCR analysis of RNAs from mouse livers. Bars, mean values and S.D. from six animals per treatment group. *, p < 0.05; **, p < 0.01.
Production of Recombinant Adenovirus Particles. Expression cassettes of interest were PCR-amplified using vector-specific primers with attB1/attB2-Gateway extensions for subsequent cloning into pDONR221 (Invitrogen). For pcDNA3 constructs (Vp16-PXR, Vp16-CAR), the following primers were used: TTAGGGTTAGGCGTTTTGCGC (forward) and TCAGAAGCCATAGAGCCCAC (reverse). Entry clones carrying the PCR products were used for cloning into pAd-DEST (Invitrogen). PacI-digested plasmids were transfected into HEK293A cells and adenovirus particles produced and processed according to the manufacturer's recommendations (Invitrogen). Functionality of PXR- and CAR-expressing adenoviruses was assessed in reporter gene assays in CV-1 cells using PXR- and CAR-responsive reporter vectors. We also tested the recombinant proteins in mouse hepatocytes by measuring mRNA expression of the target genes Cyp3a11 and Cyp2b10, respectively (data not shown). The adenovirus particles encoding recombinant forms of AMPK have been described previously (Rencurel et al., 2006).
Immunoblotting, Gel-Mobility-Shift Assay, and Chromatin Immunoprecipitation. For Western blot analysis of SREBP1, liver proteins were extracted from 100 to 200 mg of frozen tissue in 1 ml of ice-cold buffer [50 mM Tris-HCl, pH 7.4, 100 mM KCl, 1 mM EDTA, pH 8.0, 10 mM β-mercaptoethanol, 5 mM dithiothreitol, 0.1% (v/v) Triton X-100, 0.1% (v/v) Nonidet P-40, 1 tablet/50 ml buffer Protease Inhibitor Cocktail (Roche Diagnostics] in a 5-ml polystyrene tube using a Polytron Rotor-Stator Homogenizer (Kinematica, Basel, Switzerland). The homogenate was centrifuged for 30 min at 10,000 rpm at 4°C, and 50 μg of protein was loaded on a 10% SDS gel. SREBP1 isoforms were detected using mouse anti-SREBP1 monoclonal antibody (anti-SREBP1 monoclonal antibody; BD PharMingen, San Diego, CA).
Transcription factors were synthesized in vitro by using the TnT T7 Quick-Coupled Transcription/Translation System (Promega) according to the manufacturer's instructions. Probes were labeled with the Klenow fragment of DNA polymerase in the presence of radiolabeled [-32P]ATP, and the probe was purified over a Biospin 6 chromatography column. A volume of labeled oligonucleotide corresponding to 100,000 cpm was used for each reaction in 10 mM Tris-HCl, pH 8.0, 40 mM KCl, 0.05% Nonidet P-40, 6% (v/v) glycerol, 1 mM dithiothreitol containing 0.2 μg of poly(dI-dC) · poly(dI-dC) and 2.5 μl of the in vitro-synthesized proteins as described previously (Handschin et al.). The mix was incubated for 20 min at room temperature and subsequently electrophoresed on a 6% polyacrylamide gel in 0.5× Tris-borate/EDTA buffer (1× Tris-borate/EDTA buffer is composed of 0.9 M Tris-Borate and 0.002 M EDTA, pH 8.3) followed by autoradiography at 70°C. Oligonucleotides used for electrophoretic mobility shift assays were obtained as follows. For the mouse Insig-1 DR4. the following oligonucleotides were annealed and labeled using polynucleotide kinase: CCTGAGGGTCAACAGAGGACACCTAG (forward) and CTAGGTGTCCTCTGTTGACCCTCAGG (reverse).
Chromatin immunoprecipitation was performed using the EZ-Chip kit from Millipore (Billerica, MA). Primary mouse hepatocytes were infected with adenoviral particles encoding HA-tagged vp16-mouse CAR or mouse PXR, respectively. After 24 h, cells were harvested, and samples were processed according to the manufacturer's recommendations. For immunoprecipitation, HA antibody (monoclonal HA.11 clone 16B12 mouse IgG1 MMS-101P) from Covance Research Products (Princeton, NJ) was used.
Targeting of Insig-1 in Primary Mouse Hepatocytes by siR-NAs. For the transfection of primary mouse hepatocytes, Dharmafect1 transfection reagent (Dharmacon RNA Technologies (Lafayette, CO) was used. siRNAs (100 nM; siGenome SmartPool mINSIG1 and siControl Nontargeting siRNAPool; Dharmacon) and 14 μl of Dharmafect were used according to the manufacturer's instructions. Six hours after transfection, medium was removed and replaced with fresh medium without serum. Twenty-four hours later, medium was replace by medium containing 500 μM phenobarbital and mRNAs and Srebp-1 protein analyzed after 24 h as described.
Results
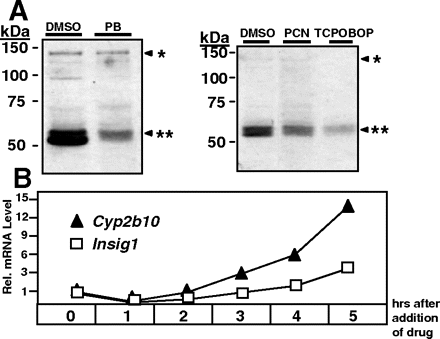
Mice were injected with drugs known to activate nuclear receptors PXR and CAR, namely PB (activator of both PXR and CAR), PCN (activator of PXR), and TCPOBOP (activator of CAR; Fig. 1). After 10 h of exposure, liver samples were analyzed for hepatic triglycerides and cholesterol (Fig. 1, top). All three drugs caused a substantial drop in triglycerides, whereas cholesterol levels were less affected. PB and TCPOBOP at the doses applied resulted in a 49 or 67% decrease in triglycerides, respectively, and a 28 or 33% decrease in cholesterol. Serum analysis of PB-treated animals revealed no significant change in triglycerides or cholesterol at 10 h (Fig. 1, top, right). RT-PCR analysis of these livers showed marked induction of Insig-1 mRNA (Fig. 1, middle). The mRNA levels of Insig-2 were not significantly induced by drug treatment (data not shown), and mRNA levels of Srebp genes remained unaffected by drug treatment as well. Accordingly, Hmg-CoA reductase, a target gene of Srebp-2 (Horton et al., 1998), was unchanged, whereas stearoyl-CoA desaturase 1, which is a target gene of Srebp-1 (Shimano et al., 1999), was reduced after drug treatment. Again, TCPOBOP showed strongest effects (Fig. 1, middle). Whether the reduction in hepatic triglycerides was due to reduced nuclear expression of Srebp-1 was tested by immunoblotting of liver protein extracts from drug-treated animals using an antibody that can discriminate between the inactive precursor form of Srebp1 and the activated nuclear (mature) form of Srebp-1 (Fig. 2A). The graph shows a reduction in nuclear content of Srebp-1 in drug-treated mice with strongest effects by PB and TCPOBOP. A time course experiment in primary mouse hepatocytes revealed that the time-dependent induction profile of Insig-1 mRNA paralleled the one of the classic CAR- and PXR-inducible gene Cyp2b10 (Fig. 2B).
Effect of drugs on Srebp1 protein levels. A, Srebp-1 immunoblot protein analysis in mouse livers after 10-h drug treatment. Mice were injected 100 mg/kg PB, 10 mg/kg TCPOBOP, or 40 mg/kg PCN in a 5% dimethyl sulfoxide-corn oil solution i.p. or with vehicle, Proteins were extracted from liver homogenates and analyzed using a monoclonal Srebp-1 antibody. *, Srebp-1 precursor form; **, Srebp-1 mature form. B, time course of Cyp2b10 and Insig-1 mRNA induction in primary mouse hepatocytes exposed to 500 μM PB.
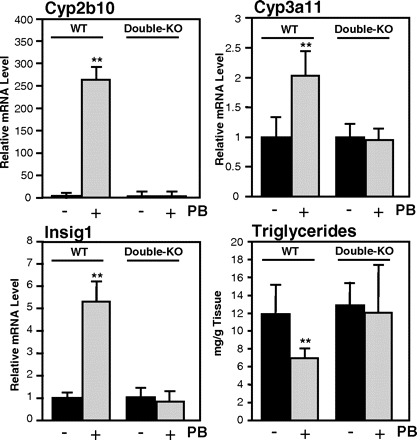
Role of CAR and PXR in the regulation of Insig-1 and hepatic triglycerides. Gene expression changes (RT-PCR) of P450s (top) and Insig-1 in CAR/PXR wild-type and PXR/CAR-null mice 10 h after injection of 100 mg/kg PB. Bars, mean values from six animals per treatment group and S.D.s therefrom. **, p < 0.01. Bottom, right, triglyceride analysis in mouse liver homogenates of the same animals (for triglyceride analysis, see Materials and Methods).
To define the role for PXR and CAR in the activation of Insig-1 and the subsequent drop in hepatic triglycerides, we applied PB to mice deficient in these two receptors (Zhang et al., 2004; Fig. 3). Figure 3 shows that two typical target genes of PXR and CAR, Cyp2b10 and Cyp3a11, expectedly were not inducible by PB in PXR/CAR-null mice. Blunted induction of Insig-1 mRNA in these animals after PB treatment as well as an unchanged triglyceride profile compared with wild-type animals was observed (Fig. 3, bottom). These data support a PXR/CAR-dependent mechanism for the triglyceride-lowering effect of inducer compounds.
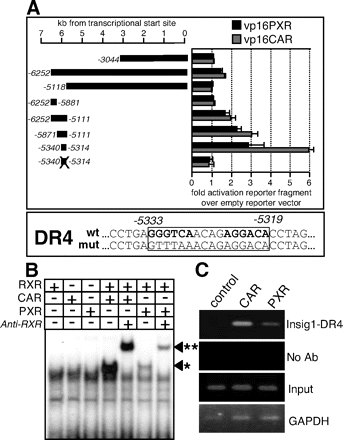
Analysis of the Insig-1 promoter for drug response elements. A, reporter gene assays using different genomic DNA sequences from the Insig-1 promoter cloned into tk-Luc vector. At the bottom of the figure, a Luc vector with a mutated Insig-1 DR-4 was used. Plasmids were cotransfected together with mouse CAR or PXR into CV-1 cells and luciferase activities determined 16 h after addition of 1 μM TCPOBOP and 10 μM PCN, respectively. Cell lysates were analyzed for luciferase expression, and -fold activation of reporter fragment was calculated relative to empty luciferase vector treated with drugs is indicated. The sequence of the identified DR-4 type drug response element as well as the mutated form used in the reporter assay is shown in a separate box. B, gel shift assay using in vitro-translated mouse CAR, PXR, and RXR and a radiolabeled oligonucleotide encoding the putative Insig-1 DR-4 element. The experimental conditions for each lane are indicated in the panel above the picture: +, corresponding receptor was added; -, no receptor was added; *, position of shifted nuclear receptor heterodimer; and **, position of anti-RXR supershifted nuclear receptor heterodimer. C, association of CAR and PXR with the Insig-1 DR-4 analyzed by chromatin immunoprecipitation in primary mouse hepatocytes using adenovirus encoding HA-tagged CAR and PXR, respectively. Lower panel shows a control experiment using the same lysates on the glyceraldehyde-3-phosphate dehydrogenase promoter, androstenol (AND), TCPOBOP, and PCN.
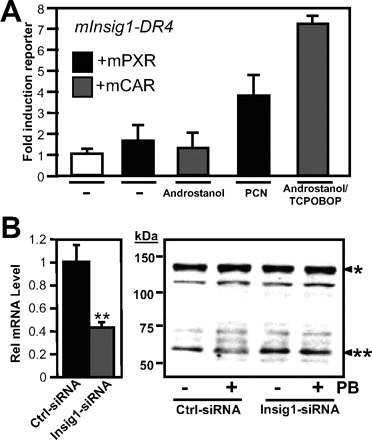
We therefore designed experiments to identify functional binding sites for the PXR and CAR in the regulatory region of Insig-1. Two 3-kb fragments of genomic DNA from the 5′-flanking region of the Insig-1 gene were cloned into a luciferase reporter vector, and activity was assessed in transactivation assays in CV-1 cells (Fig. 4A). The first DNA element spanned the transcriptional start site, including the proximal promoter of Insig-1 to -3044 bp, and showed no activation after drug treatment. The second large DNA stretch was overlapping with the first one and ended at -6252 bp. There was slight (as compared with empty control luciferase vector) activation of reporter gene transcription after drug treatment. This DNA element was cut into smaller pieces and activity assessed until a 760-bp fragment revealed a robust response to PXR and CAR. Within this fragment, a DR-4-type drug response element was identified using the Nubiscan algorithm (Podvinec et al., 2002). This element responded well to PXR and CAR, and specificity was assessed using a mutated version of the DR-4 element, which resulted in a decreased response to drugs (Fig. 4A). The same DR-4 element was used in electromobility shift assays together with in vitro-translated PXR, CAR, and their heterodimeric partner, RXR (Fig. 4B). A strong band was observed when CAR and RXR were incubated with the oligonucleotide carrying the DR-4 element, and less intensive binding appeared when PXR was used. Both nuclear receptor complexes were supershifted by coincubation with an anti-RXR antibody. Functionality of this element was tested in vivo by chromatin immunoprecipitation in primary mouse hepatocytes (Fig. 4C). Cells were infected with adenovirus particles expressing HA-tagged versions of both CAR and PXR. The amplified PCR product corresponds to the 157-bp region in the murine Insig-1 promoter where the designated DR-4 element is located. In Fig. 5B, a reporter gene analysis using the Insig-1 DR-4 with inducers of mouse PXR and CAR, respectively, was performed and revealed a 4-fold induction after PCN treatment and a 7.2-fold induction after TCBOBOP treatment (Fig. 5A).
A role for Insig-1 in mediating the repressive effects of inducer drugs was established by siRNA-mediated inhibition of Insig-1 expression and concurrent treatment with PB (Fig. 5B). Primary mouse hepatocytes were transfected with either unspecific control siRNAs or siRNAs targeting Insig-1. After 48 h, mRNA analysis revealed markedly reduced Insig-1 expression (Fig. 5B, left). Immunoblot analysis of Srebp-1 protein levels in siRNA-transfected cultures treated with or without PB was performed (Fig. 5B, right). The results reveal reduced nuclear expression of Srebp-1 in cells transfected with control siRNAs and treated with PB. In cells where Insig-1 expression was reduced by siRNAs, Srebp-1 protein levels in the nucleus remained unaffected (Fig. 5B, right).
Induction of Insig-1 DR-4 reporter gene by PXR and CAR inducers and lack of effect of phenobarbital on SREBP-1 proteins when Insig-1 expression is suppressed by RNAi. A, effect of PXR, CAR, and their ligands/activators on the Insig-1 DR-4 reporter gene. PXR was activated by overnight treatment with 10 μM PCN. Constitutive active CAR was repressed by the addition of 1 μM androstenol and activated by addition of 10 μM TCPOBOP. -Fold activations are calculated as relative reporter gene levels over nondrug-treated cells. B, primary mouse hepatocytes were transfected with either control siRNAs or siRNAs against Insig-1. Left, mRNA analysis of Insig-1-siRNA effect after 48 h; right, effect of PB treatment on Srebp-1 protein levels in cultures transfected with either control or Insig-1 siRNA. *, precursor of Srebp-1; **, mature form of Srebp-1.
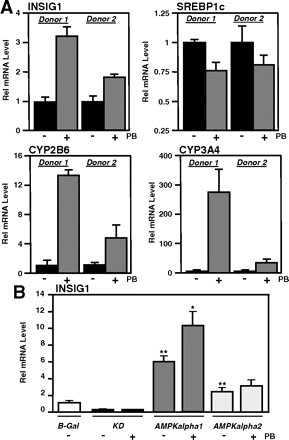
Induction of Insig-1 by PB and AMPK in primary human hepatocytes. A, cells from two different donors were incubated with 500 μM PB, and mRNA was analyzed 50 h thereafter. B, AMPK and PB synergistically activate human INSIG-1. Primary human hepatocytes from a third donor were infected with adenoviral particles encoding the control gene (B-Gal), a dominant-negative form of AMPK [kinase dead (KD)], the α-1 subunit of AMPK (AMPKα1), or the α-2 subunit of AMPK (AMPKα2). *, p < 0.05; **, p < 0.01; n = 3; p values were calculated between either untreated or treated wells.
To reveal a potential role of this mechanism in humans, we tested the inducibility of human Insig-1 by PB in primary human hepatocytes (Fig. 6A). Treatment for 50 h with PB resulted in a significant induction of Insig-1 in cultures of two different donors, and this was paralleled by a reduction in Srebp1c expression. Induction of CYP2B6 and CYP3A4 served as positive controls.
As recently reported, induction of drug-metabolizing enzymes requires activation of AMP-activated kinase (Rencurel et al., 2005, 2006). We therefore wanted to test whether this kinase, alone or in combination with drug, can regulate transcription of Insig-1 (Fig. 6B). Primary human hepatocytes were infected with control virus (expressing β-galactosidase) or different versions of AMPK: a dominant-negative construct (kinase dead), the α-1 or the α-2 subunit of AMPK. Although the dominant-negative version repressed expression of Insig-1, both subunits induced Insig-1 with stronger effects seen using the α-1 subunit. Furthermore, exposure to PB enhanced these effects (Fig. 6B).
Discussion
The experiments described here reveal a novel molecular mechanism by which drugs that induce drug-metabolizing enzymes and drug transporters can acutely regulate hepatic triglyceride levels. Although several P450s and other enzymes involved in metabolism and transport of xenochemicals have been known to be targets of nuclear receptors CAR and PXR, the up-regulation of Insig-1 by the same receptors after drug treatment is new and adds a potentially clinically important aspect to the present understanding on how the liver reacts to accumulating lipophilic compounds such as PB. This report shows that the drug-metabolizing process also includes direct regulation of hepatic lipid biosynthesis by induction of an important regulatory protein, as is Insig-1. We present evidence for a functional DR-4 binding site for CAR and PXR in the upstream promoter region of Insig-1 (Fig. 4). Binding of the xenobiotic receptors to this DR-4 site can account for the induction of Insig-1, which results in the reduced expression of the activated nuclear form of Srebp-1 and the substantial reduction in hepatic triglycerides after only 10 h of treatment (Figs. 1 and 2). The fact that Insig-1 has been observed in expression studies to be induced early after treatment with the CAR ligand TCPOBOP (Locker et al., 2003) and that overexpression of Insig-1 in livers of mice has been shown to cause a drop in triglyceride levels with smaller effects on cholesterol (Engelking et al., 2004; Takaishi et al., 2004) made Insig-1 an interesting candidate gene for transcriptional regulation by CAR and PXR. We first established that in CAR/PXR double-knockout mice, PB had lost its effect on triglycerides, and there was no induction of Insig-1 (Fig. 3). This strongly supported the idea of a CAR/PXR-mediated transcriptional activation of the Insig-1 gene. Moreover, the induction of Insig-1 mRNA appeared early after the addition of drug (Fig. 2B), making a rapid decrease in the activated nuclear form of Srebp-1 protein levels a plausible scenario (Fig. 2A). The more pronounced effects on triglycerides seen with the CAR activators TCPOBOP and PB compared with the PXR ligand PCN are in line with the more pronounced activation of the Insig-1 promoter by CAR (Figs. 4A and 5B), with the higher affinity of CAR to bind to the DR-4 element (Fig. 4B) as well as with the stronger enrichment in CAR-immunoprecipitated samples of this fragment (Fig. 4C). A role for Insig-1 in mediating these effects was established in mouse hepatocytes with siRNA-reduced Insig-1 expression (Fig. 5B). Although the repressive effect of PB on nuclear protein levels of Srebp-1 was evident, in cells with repressed Insig-1 expression, this effect was not detectable, strongly supporting the concept that PXR/CAR-activated Insig-1 is responsible for reduced Srebp-1 and thereby for lowered hepatic triglycerides. Moreover, the fact that these effects could be reproduced in human hepatocytes supports the concept of a general mechanism by which drugs affect hepatic lipid biosynthesis (Fig. 6A). Insig-2, the other member of the Insig family of Srebp-regulating genes, was not affected by PXR/CAR inducers, and a potential role of this gene in linking drug treatment to reduced triglyceride levels was not pursued. It cannot be ruled out, of course, that under different conditions, this gene may also play a role in mediating lipid synthesis due to a xenobiotic challenge.
An effect of PXR on lipogenic genes has recently been described by Nakamura et al. (2007). In strong support of our data, they observed down-regulation of lipogenic genes by the PXR-specific activator PCN, and this effect was not seen in PXR-/- mice. Interestingly, Nakamura et al. furthermore observed an induction of stearoyl-CoA desaturase 1 in PCN-treated animals that were fasted for 24 h. This suggests an additional level of regulation of lipogenesis by xenobiotics in the fasted state.
Another new finding of our study is the role of AMPK in the induction of Insig-1 (Fig. 6B). AMPK is considered a metabolic master-switch sensing cellular energy levels and regulating glucose transport and gluconeogenesis. It is activated in response to metabolic stress signals that deplete cellular ATP and stimulate fatty acid oxidation (Kahn et al., 2005). It was recently shown that CAR-dependent induction of CYP2B by PB requires activation of AMPK (Rencurel et al., 2006). Blättler et al. (2007) demonstrated that PB interferes with mitochondrial function and activates the AMPK upstream kinase LKB1, which then mediates the activation cascade of AMPK to CAR. Interestingly, AMPK, either via activation by 5-aminoimidazole-4-carboxamide riboside or via adenoviral overexpression of its catalytic subunit, also has been shown to reduce Srebp-1c expression (Zhou et al., 2001; Foretz et al., 2005). Because these observations lacked a mechanistic explanation, the activation of Insig-1 by AMPK shown here may indicate a signaling pathway leading to repression of Srebp-1c. In line with observations by Rencurel et al. (2006), AMPK alone seems capable of regulating expression of CAR/PXR target genes, and addition of a nuclear receptor activator leads to a synergistic effect on gene transcription. However, the detailed interplay among PB, nuclear receptors, and AMPK in the induction of Insig-1 clearly requires further investigation.
In addition, the data presented here account for the immediate physiologic response of the liver to a xenobiotic challenge. Long-term treatment with drugs leading to constant activation of PXR and/or CAR may lead to diverse adaptive gene regulations to maintain lipid homeostasis (Kiyosawa et al., 2004; Zhou et al., 2006).
In conclusion, the results of our experiments suggest that the signaling pathways involved in mediating the effect of xenobiotics on detoxification also induce Insig-1, a gene regulating lipid biosynthesis, and that this is associated with an acute lowering of triglyceride levels in the liver. Because Insig-1 has recently been suggested as a possible drug target for the treatment of dyslipidemic diseases including diabetes (Nakagawa et al., 2006), the observation that CAR and PXR ligands or activators induce Insig-1 may have clinical consequences and explains the reported alterations in lipid levels after drug therapy.
Acknowledgments
We thank Michael Podvinec (Institute of Bioinformatics, University of Basel, Switzerland) for assistance with in silico sequence analysis and Frederic Delobel (Hoffman-La Roche) for experimental support with primary mouse hepatocytes.
Footnotes
-
This study was supported by grants from the Swiss National Science Foundation and the STEROLTALK project (EC Contract LSHG-CT-2005-512096) under the 6th framework program.
-
ABBREVIATIONS: P450, cytochrome(s) P450; PXR, pregnane X receptor; CAR, constitutive androstane receptor; RXR, retinoid X receptor; ER, endoplasmic reticulum; Srebp, sterol regulatory element binding protein; PB, phenobarbital; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]-benzene; PCN, pregnenolone-16α-carbonitrile; RT, reverse transcriptase; PCR, polymerase chain reaction; HA, hemagglutinin; AMPK, AMP-activated kinase; siRNA, small interfering RNA; bp, base pair(s).
- Received August 17, 2007.
- Accepted January 4, 2008.
- The American Society for Pharmacology and Experimental Therapeutics