Abstract
Heat shock proteins (Hsp) are a class of stress-inducible proteins that mainly act as molecular protein chaperones. This chaperone activity is diverse, including assisting in nascent protein folding and regulating client protein location and translocation within the cell. The main proteins within the Hsp family, particularly Hsp70 and Hsp90, also have a highly diverse and numerous set of protein clients, which when combined with the high expression levels of Hsp proteins (2%–6% of total protein content) establishes these molecules as “central regulators” of cell protein physiology. Among the client proteins, Hsps regulate numerous signal-transduction and receptor-regulatory kinases, and indeed directly regulate some receptors themselves. This also makes the Hsps, particularly Hsp90, central regulators of signal-transduction machinery, with important impacts on endogenous and drug ligand responses. Among these roles, Hsp90 in particular acts to maintain mature signaling kinases in a metastable conformation permissive for signaling activation. In this review, we will focus on the roles of the Hsps, with a special focus on Hsp90, in regulating receptor signaling and subsequent physiologic responses. We will also explore potential means to manipulate Hsp function to improve receptor-targeted therapies. Overall, Hsps are important regulators of receptor signaling that are receiving increasing interest and exploration, particularly as Hsp90 inhibitors progress toward clinical approval for the treatment of cancer. Understanding the complex interplay of Hsp regulation of receptor signaling may provide important avenues to improve patient treatment.
Introduction
The heat shock proteins (Hsp) are a conserved class of stress-inducible proteins that primarily act as molecular chaperones (Odunuga et al., 2004; Li and Buchner, 2013). This chaperone activity is diverse, with the central Hsp regulators Hsp70 and Hsp90, assisted by cochaperones such as Hsp70-Hsp90 organizing protein–stress-induced phosphoprotein-1 (Hop/STIP1) and Cdc37, performing a central role in promoting proper folding of nascent polypeptides (Odunuga et al., 2004; Gould et al., 2009; Assimon et al., 2013; Li and Buchner, 2013; Verba et al., 2016). However, the chaperone activity of the Hsps extends well beyond the promotion of proper protein folding. Hsp90 in particular maintains mature signaling kinases and other signal-transduction proteins in the proper metastable conformation necessary for signaling activation (Li and Buchner, 2013). Hsp90 can also regulate the location and translocation of signaling kinases and other proteins within the cell, helping to maintain the proper organization of cell biology and signal transduction (Yang et al., 2012). Hsp90 can also act as a molecular scaffold, selectively associating signaling kinases and other proteins in close proximity for selective activation during signal transduction (Schulte et al., 1995; Jaiswal et al., 1996; Nieto-Miguel et al., 2008). Other Hsps show a diverse set of roles in regulating cell function, such as Hsp27 acting as a central regulator of actin polymerization and thus cell motility and other cytoskeletal functions (Okada et al., 2005; McConnell and McAlpine, 2013).
The Hsps perform these different chaperone functions on a broad array of client proteins. These client proteins span nearly every functional class, including transcription factors, nuclear hormone receptors, viral proteins, and signaling kinases. In this review, we will focus on the role of Hsps, particularly Hsp90, in regulating receptor-related signaling. Of the receptors in the genome, G protein-coupled receptors (GPCRs) constitute the largest class, with more than 800 GPCRs in humans; these represent a plurality of the drug targets exploited in the current pharmacopeia and in ongoing clinical trials (Hauser et al., 2017). As such, GPCRs represent the largest area of study for receptor activation and signal transduction and will be the primary but not exclusive focus of this review.
Heat Shock Protein 90
G Protein-Coupled Receptor Kinases.
One of the largest classes of signaling proteins regulated by Hsp90 are signaling kinases, and regulation of these kinases is the main means by which Hsp90 regulates receptor signaling. Hsp90 regulates a broad swathe of these kinases across numerous functional classes, often in concert with its kinase-specific cochaperone Cdc37 (Grammatikakis et al., 1999; Tatebe and Shiozaki, 2003; Gould et al., 2009; Ota et al., 2010; Li and Buchner, 2013; Verba et al., 2016). A selection of these regulated kinases are listed in Table 1, and further information on Hsp90 client proteins can be found in a web database maintained by Didier Picard of the University of Geneva (www.picard.ch) as well as the following references: Tsaytler et al. (2009); Echeverría et al. (2011). Of these client proteins, the G protein-coupled receptor kinases (GRKs) are the most closely associated with GPCR signaling, as GRKs directly phosphorylate GPCRs upon activation to promote desensitization, β-arrestin recruitment, and internalization (Brackley et al., 2016; Penela, 2016).
Selected signaling kinase families regulated by Hsp90
Selected families of signaling kinases known to interact with and are regulated by Hsp90 are shown. The list is not exhaustive. From Echeverría et al. (2011) and a site maintained by Dr. Didier Picard of the University of Geneva (www.picard.ch).
Hsp90 has been shown to stabilize and promote the maturation of GRKs 2, 3, 5, and 6, through which Hsp90 can generally promote GPCR desensitization and internalization across a broad array of GPCR targets (Luo and Benovic, 2003; Wu et al., 2012; Penela, 2016). This interaction with GRK3 was found to be regulated by acute calcium release in neuroblastoma cells, suggesting that Hsp90 could acutely regulate GRK3 activity in activated neurons (Salim and Eikenburg, 2007). Hsp90 also regulates GRK localization and transport within the cell. Hsp90 was found to target GRK2 to the mitochondria in response to extracellular signal-regulated kinase (ERK)–mitogen-activated protein kinase (MAPK) activation during ischemic stress; this translocation was found to promote the mitochondrial permeability transition and cell death (Chen et al., 2013a). These results suggest that Hsp90 inhibitors could be potential therapies for ischemia-reperfusion injury after myocardial infarction or stroke, which was supported by a recent experimental stroke study (Kim et al., 2015). These combined studies suggest that Hsp90 could have a broad impact on GPCR signaling via regulation of GRKs.
Heterotrimeric G Proteins.
The heterotrimeric G proteins are a class of effector proteins that convey signal transduction downstream of all GPCRs. These proteins form an obligate trimer of an α-subunit that differentially modulates cAMP or calcium signaling depending on subtype, and a β/γ-dimer that evokes phospholipase C/calcium signaling (Gurevich and Gurevich, 2017). Regulation of these G proteins by Hsp90 thus has the potential to regulate signal transduction downstream of up to hundreds of GPCRs. Hsp90 was shown to direct mature Gα12 (but not Gα13) specifically to lipid rafts and the mitochondria, which is required for Gα12 signaling; this demonstrates an obligate role for Hsp90 in all GPCR signaling evoked by this protein (Vaiskunaite et al., 2001; Waheed and Jones, 2002; Andreeva et al., 2008; Montgomery et al., 2014). Among other potential roles, this Gα12 targeting was shown to be necessary for tight junction formation in Mardin-Darby canine kidney cells (Sabath et al., 2008). Hsp90 was also shown to play a similar role in translocation and targeting of the Gαi2 protein to the cannabinoid type-2 receptor, through which Hsp90 could have a broad impact on cannabinoid signaling in the central nervous system and immune system (He et al., 2007). Hsp90 was further shown to regulate guanosine di/triphosphate (GDP/GTP) exchange of Gαs by forming a complex with this protein; as GDP/GTP exchange is central to G protein signaling (Gurevich and Gurevich, 2017), Hsp90 could have a broad impact on all Gαs-coupled GPCRs through this interaction (Gibbs et al., 2009). Although less specific than the above regulatory roles, Hsp90 was also shown to maintain protein expression of Gαo, through which Hsp90 could generally promote GPCR signaling of receptors that couple to this G protein (Busconi et al., 2000). Intriguingly, Hsp90 was also shown to form a complex with the Gβ/γ-dimer; although this interaction could broadly impact GPCR signaling through Gβ/γ, no functional impact has yet been identified for this association (Inanobe et al., 1994).
Small GTPases.
The small GTPases (Ras, Rac, etc.) are a large class of downstream signaling molecules that are highly impactful in numerous signal-transduction cascades, including GPCR signaling as well as other receptor families. This family of proteins, although GTPases that cycle GDP with GTP, are distinct from the heterotrimeric G proteins above. The small GTPase Ras is perhaps the best studied Hsp90 client protein, as increased Ras activity owing to mutation or other causes is a major driver of cancer cell proliferation through the Ras-Raf–MEK–ERK-MAPK cascade [for examples, see Haarberg et al. (2013), Bar et al. (2017), and Park et al. (2017)]. Treatment with Hsp90 inhibitors to block cancer cell growth can act to destabilize Hsp90-Ras interactions directly, or circumvent the pro-proliferation drive of Ras.
Hsp90 has been shown to complex with Raf kinase to promote cell proliferation, and this interaction has further been shown to be dependent on Ras. Ras signaling promotes Hsp90-Raf formation, which in turn associates the Hsp90-Raf complex with Ras in the membrane. This trinary complex acts as a positive feedback interaction to promote Ras-Raf signaling and thus cell proliferation [references for all above claims: Schulte et al. (1995); Cissel and Beaven (2000); Mitra et al. (2016); Diedrich et al. (2017)]. Interestingly, Hsp90 has also been shown to repress Ras-mediated protein kinase A signaling, demonstrating the importance of context in the actions of Hsp90 to regulate signaling (Shapiro et al., 2009).
Beyond Ras, Hsp90 has been shown to associate with and regulate several members of the Rab family. Hsp90 was shown to regulate the access of Rab to the protein α-GDP dissociation inhibitor (α-GDI), which regulates GDP/GTP exchange. As such, this interaction represents an important signal-responsive mechanism to regulate Rab activity (Sakisaka et al., 2002; Chen et al., 2005; Chen and Balch, 2006; Raffaniello et al., 2009). Hsp90 was also shown specifically to complex with Rab11 and Rab5, through which Hsp90-regulated signaling-responsive endocytosis and sorting (Liu et al., 2009; Allonby et al., 2014; Bozza et al., 2014). Hsp90 likewise targets Rab3 to the membrane to regulate internalization (Chen et al., 2013b). Interestingly, some Rab proteins have been shown to regulate Hsp90, in that Rab27 has been shown to regulate the cleavage and secretion of Hsp90 as an extracellular progrowth signal (Hendrix et al., 2010).
Hsp90 has also been shown to link Rho signaling directly to the GPCR vascular endothelial growth factor receptor. Hsp90 couples RhoA to the vascular endothelial growth factor receptor and is necessary for the activation of focal adhesion kinase [FAK; Le Boeuf et al. (2004)]. This makes Hsp90 crucial for vasculogenesis signaling via VEGF. Hsp90 also regulates cytoskeletal remodeling via Rho/RhoC, relating to motility, with Hsp90 blocking cancer migration in some contexts through the regulation of Rho (Amiri et al., 2007; Willmer et al., 2013). Hsp90 also regulates access to Rho of the Rho-GDP exchange regulatory protein Vav3, through which Hsp90 may regulate a broad swathe of GPCR and other receptor signaling through Rho (Wu et al., 2013). Finally, Hsp90 increases Src-mediated RhoA signaling in endothelial cells, implicating Hsp90 in the promotion of macrophage extravasation and edema in response to inflammation (Joshi et al., 2014).
Continuing with this theme, Hsp90 also regulates the Rac family mostly in the context of inflammation. Hsp90 complexes with Rac1 to promote its signaling, which was found to be crucial in the innate immunity response (Thao et al., 2007). Likewise, Hsp90 promotes Rac1 signaling in infected gastric epithelial cells, promoting reactive oxygen species generation and cell damage in this context (Cha et al., 2010). This could make Hsp90 inhibitors useful for the treatment of gastric ulcers. Finally, Hsp90 was shown to promote a Rac1–PP5–ERK-MAPK complex, thereby regulating ERK-MAPK activation and activity (Mazalouskas et al., 2014). Together, these studies demonstrate the strong impact of Hsp90 on these small GTPases, which will have broad effects on the signaling of multiple receptor families in multiple physiologic contexts.
Signaling Kinases.
Hsp90 also regulates an incredibly broad swathe of signaling kinases, by which receptors carry out much of their signaling activity [Table 1, Tsaytler et al. (2009; Echeverría et al. (2011)]. We cannot exhaustively cover each kinase family but will highlight below some prominent examples to demonstrate the impact of Hsp90 on receptor-kinase signaling. By regulating these kinases, Hsp90 may regulate the signaling of every receptor and every physiologic process they are implicated in.
The ERK-MAPK pathway is perhaps the best studied in regard to Hsp90 regulation, owing to the importance of this pathway in regulating cell survival and proliferation, and thus a major mechanism for Hsp90 inhibition in cancer therapy (Sidera and Patsavoudi, 2014; Nagaraju et al., 2016; Park et al., 2017). As discussed above, Hsp90 forms a complex with the small GTPase Ras and the kinase Raf that promotes their activity, both of which go on to activate ERK-MAPK (Schulte et al., 1995; Cissel and Beaven, 2000; Mitra et al., 2016; Diedrich et al., 2017).
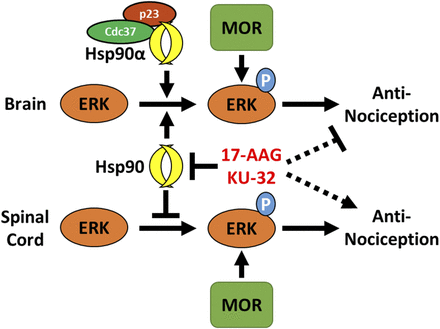
However, Hsp90 has also been directly associated with ERK signaling, which has been shown to be dependent on context, that is, increased in some contexts and decreased in others. This theme is present in work relating to the opioid receptors. Hsp90 has only been lightly studied in opioid receptor signaling: Hsp90 inhibitors were shown to block opioid cAMP superactivation in a cell model (Koshimizu et al., 2010), and chronic morphine treatment was shown to increase Hsp90 expression in the synapse, whereas Hsp90 inhibitors acutely blocked morphine withdrawal behavior (Abul-Husn et al., 2011). In our own work, we found that Hsp90 strongly increases ERK-MAPK signaling in the brain, leading to loss of ERK activation and morphine analgesia in some pain states with Hsp90 inhibitor treatment (Lei et al., 2017). In contrast, we found that Hsp90 in the spinal cord strongly potentiates ERK-MAPK signaling, in that Hsp90 inhibitor treatment in the spinal cord led to enhanced ERK activation and analgesia in response to morphine (unpublished data, Fig. 1). These results demonstrate how context determines the role of Hsp90 regulation of ERK signaling, with strongly divergent physiologic consequences. This theme is echoed in the literature: Hsp90 complexed with and promoted ERK-MAPK signaling in some contexts (Sétáló et al., 2002; Georgakis et al., 2006; Rice et al., 2008; Yun et al., 2011; Wang et al., 2014) but blocked it in others (Lin et al., 2015).
Summary of the context-dependent role of Hsp90 in regulating ERK-MAPK in the brain vs. spinal cord. The μ opioid receptor (MOR) promotes ERK-MAPK phosphorylation in response to opioid signaling, which induces antinociception in pain states. Hsp90 differentially regulates this process by promoting ERK-MAPK activation in the brain and repressing it in the spinal cord. Hsp90 inhibitors (17-AAG, KU-32[Ansar et al., 2007; Lei et al., 2017]) or cochaperone inhibitors (gedunin, celastrol[Sassa et al., 1990; Brandt et al., 2008]) reverse this regulation, leading to blocked ERK-MAPK activation in the brain and enhanced activation in the spinal cord; this results in blocked opioid antinociception in the brain and enhanced antinociception in the spinal cord (dashed lines). Using selective inhibitors and in vivo CRISPR, we further found that Hsp90α and the cochaperones Cdc37 and p23 mediate this regulation in the brain. Data taken from Lei et al. (2017) and unpublished data from the Streicher laboratory.
Beyond the MAPKs, Hsp90 also regulates numerous other influential signaling kinase families that signal downstream of receptors (Table 1). These include protein kinase C, a central regulatory kinase in the cell; Hsp90 was shown to target protein kinase C ε to the mitochondria after adenosine receptor activation, leading to ischemic protection in the brain and heart (Yang et al., 2012; Thompson et al., 2013). Hsp90 also generally promotes stability and activation of the cyclin-dependent kinases, which are crucial for regulating the cell cycle; this promotion may underlie in part the mechanism by which Hsp90 promotes cancer growth, although in at least some contexts Hsp90 prevents inappropriate cell cycle entry by the cyclin-dependent kinases (Mikolajczyk and Nelson, 2004; Chaklader et al., 2012; Stetz et al., 2017). Another central kinase regulated by Hsp90 is glycogen synthase kinase-3 (GSK-3), which requires Hsp90 for functional maturation, and which also requires Hsp90 for complexing with the Wnt/β-catenin cascade to promote signaling through that pathway (Lochhead et al., 2006; Cooper et al., 2011; Jin et al., 2016). These examples give the flavor of the wide-ranging and impactful regulation of receptor-activated signaling kinases by Hsp90 and show how crucial this protein is for regulating receptor signal transduction in numerous contexts.
Heat Shock Protein 70
Hsp70 is a close partner of Hsp90 in the protein folding and maturation process; however, Hsp70 acts earlier on in the process, so it has a smaller role in regulating mature protein activation, signaling complex formation, and subcellular targeting than does Hsp90 (Li and Buchner, 2013). In addition, treatment with typical ATP binding pocket–targeted Hsp90 inhibitors releases heat shock factor-1 (HSF-1), leading to increases in Hsp70 protein expression; this can make disentangling Hsp90 inhibition from Hsp70 induction difficult in practice (McConnell and McAlpine, 2013; Kim et al., 2015; Lei et al., 2017). Nonetheless, clear evidence has emerged for the role of Hsp70 in regulating GPCR signaling. An early study demonstrated that Hsp70 interacts with unglycosylated versions of the angiotensin receptor, leading to retention in the endoplasmic reticulum; this mechanism is thus important for insuring the transport of mature, glycosylated receptor to the membrane (Lanctôt et al., 2006). Similar studies found that Hsp70 was important in the folding and trafficking of the adenosine A2A receptor (Bergmayr et al., 2013) and the lysophosphatidic acid receptor (Zhao et al., 2014) in the endoplasmic reticulum. Continuing the theme of trafficking and internalization, Hsp70 was also found to be a substrate of GRK5, and phosphorylation by GRK5 was necessary for Hsp70 to induce agonist-mediated internalization of the chemokine receptor CXCR4 (Barker and Benovic, 2011). These studies thus demonstrate a clear role for Hsp70 in processing and targeting GPCRs specifically to the membrane, as well as internalizing those receptors after agonist activation, a crucial negative feedback loop in GPCR signaling. Interestingly, another study showed that Hsp70 could directly complex with the mature adenosine A2A receptor; this prevented G protein binding to activated receptor and attenuated receptor signaling (Lim et al., 2013). These results show that Hsp70 can dynamically regulate mature GPCR signaling. Finally, Hsp70 can also promote the expression and maturation of numerous signaling kinases, as does Hsp90. However, in general, this role appears to be weighted to simple maturation/expression and not mature kinase activation, complex formation, and cellular targeting (e.g. Hao et al., 2018).
Heat Shock Protein 40
Hsp40 is also involved in the early stages of protein maturation and folding and regulates the ATPase activity and substrate binding of Hsp70 (Li and Buchner, 2013; McConnell and McAlpine, 2013). However Hsp40 has also been shown to possess unique roles in regulating receptors. In one case, Hsp40 was found to be crucial for the proper processing and targeting of the rhodopsin GPCR within the photoreceptors. Interestingly, this role was shown to be independent of Hsp70 (Chapple and Cheetham, 2003). Likewise, Hsp40 was shown to properly process and target the human cannabinoid receptor type-1, albeit in a bacterial expression host (Skretas and Georgiou, 2009). Hsp40 also acts to regulate parts of the downstream signaling machinery. Hsp40 complexes with Hsp90 in the study discussed in the “Small GTPases” section that regulates access of α-GDP dissociation inhibitor to the small GTPase Rab, regulating the GTPase and thus signaling activity of this protein (Sakisaka et al., 2002). Likewise, Hsp40 acts to regulate the GTP hydrolysis rate of GαS proteins, through which Hsp40 could regulate a broad array of GPCR signaling (Gibbs et al., 2009). Hsp40 was also shown to respond in a dynamic way to chronic morphine treatment in the brain; Hsp40 was downregulated in the synapses whereas Hsp90 was upregulated, and Hsp90 upregulation was involved by an unknown mechanism in the development of morphine dependence and withdrawal (Abul-Husn et al., 2011). Another intriguing experiment showed that Hsp40 acts as an adaptor protein to form a trinary complex with Hsp70 and the urokinase receptor; this interaction facilitated receptor signaling and subsequent changes in cell migration, invasion, and adhesion (Lin et al., 2014).
Heat Shock Protein 27
Hsp27 is an important small Hsp that is perhaps best known as a regulator of actin polymerization; it is phosphorylated by kinases like mitogen-activated protein kinase–activated protein kinase 2 (MAPKAP-K2) (Streicher et al., 2010). Most Hsps like Hsp90 act in large part as multitarget protein chaperones that regulate protein expression, function, and localization; in contrast, Hsp27 appears to act as a more traditional signal-transduction effector. It is phosphorylated by kinases downstream of multiple identified receptors and subsequently activates other signaling molecules or changes cell physiology, as in its role in actin polymerization. It has been identified in numerous cell processes, with an identified role particularly in promoting cancer cell survival. The above general information is reviewed in Singh et al. (2017). Hsp27 has been particularly linked to tumor growth factor (TGF)-β receptor stimulation; Hsp27 was found to be responsible for cisplatin resistance and lung cancer cell survival subsequent to TGF-β treatment (Huang et al., 2017). Hsp27 was also found to regulate lung fibroblast differentiation in response to TGF-β via activation of Smad3 and ERK-MAPK (Wang et al., 2017). Other receptor systems have also been implicated in Hsp27 signaling: These include the membrane-associated androgen receptor (Li et al., 2018) and endothelin-1 stimulation (Fujita et al., 2018). Hsp27 has further been implicated in different physiologic processes downstream of these receptors. These include chemokine (C-C motif) ligand 2–induced platelet function (Liu et al., 2018), tumor necrosis factor α–induced myofibroblast migration (Saini et al., 2016), A1 adenosine receptor–induced protection against intracerebral hemorrhage (Zhai et al., 2016), and intestinal epithelial cell cytoprotection after exposure to the bacterial chemotactic peptide fMLP (Carlson et al., 2007). A number of papers have also linked Hsp27 to the β2-adrenergic receptor and its downstream signaling regulator β-arrestin 2; stimulation of this receptor led to Hsp27 induction, which can be cytoprotective in both brain and heart (Imura et al., 1999; Rojanathammanee et al., 2009; Xu et al., 2011; Martínez -Laorden et al., 2012). Care must be taken in interpreting these results, however, in that β2-adrenergic stimulation can lead to pathophysiology such as cardiomyopathy (Xu et al., 2011), and some of the above studies did not disentangle the potential protective effects of Hsp27 induction from the harmful effects observed with β2-adrenergic stimulation. Finally, Hsp27 has been linked to some of the other molecular signaling cascades discussed in the above sections. In particular, the small GTPase Ras was found to block activation of Hsp27 via reducing p38 MAPK stimulation after VEGF activation (Sawada et al., 2015). Overall, these findings indicate that Hsp27 has an important role in the regulation of receptor signal transduction via a mechanism distinct from the other Hsps discussed above.
Conclusions and Future Directions
The literature review here is not comprehensive and is merely meant to give a general overview and selection of the impact of Hsps on signal transduction. The literature on these central cell regulators is extensive and covers hundreds of client proteins, receptor systems, and physiologic contexts. The literature on these regulators will continue to grow, aided by the extensive efforts to develop different Hsp and cochaperone inhibitors for cancer and other therapies. What is clear is that these proteins have an enormous role in regulating signal transduction, which will give us important avenues to manipulate different receptor systems to achieve improved therapeutic outcomes.
In one sense, the Hsps are not good drug targets, owing to their numerous client interactions, ubiquity, and high level of expression. Despite these apparent limitations however, Hsp inhibitors are surprisingly well tolerated. In the case of Hsp90, although it is true that early geldanamycin derivatives failed clinical trials owing to liver toxicity, this effect has not been seen with newer generations of inhibitors, suggesting the early toxicity was drug- and not target-related (McConnell and McAlpine, 2013; Jhaveri et al., 2014; Sidera and Patsavoudi, 2014). In particular, the newest class of Hsp90 inhibitors are derived from a novobiocin scaffold and target the C-terminal region instead of the ATP binding pocket; these inhibitors show a prosurvival effect in neurons with beneficial effects in chronic neuropathic pain with low or no toxicity, pointing the way to new and effective Hsp therapeutics (Burlison et al., 2006; Ansar et al., 2007; Lu et al., 2009; Urban et al., 2010; Samadi et al., 2011).
Another method to effectively target the Hsps is demonstrated by Gestwicki and colleagues, who selectively targeted the cochaperone interactions of Hsp70 in cataract models to more specifically and selectively modulate Hsp proteins (Assimon et al., 2013; Assimon et al., 2015). Along these lines, Brian Blagg and colleagues have pioneered the development of isoform-selective Hsp90 inhibitors (Tash et al., 2008; Liu et al., 2015; Mishra et al., 2017), and Blagg and others have developed cochaperone-selective inhibitors like celastrol and gedunin (Brandt et al., 2008; Zhang et al., 2008). These approaches may reduce side effects and increase tolerability of Hsp-directed therapies by targeting the specific regulators of the Hsp machinery more selectively by tissue distribution and signaling process.
A more complete understanding of the role of Hsps in regulating different aspects of receptor signaling will increase our understanding of these crucial cell regulators and will also provide more targets for new therapies. The new drug approaches highlighted above combined with other potential drug therapies, such as biased agonism (Urban et al., 2007), may provide new means to use this knowledge to our advantage. These opportunities will only increase as the literature on Hsp regulation of signal transduction grows.
Footnotes
- Received September 20, 2018.
- Accepted January 12, 2019.
The author acknowledges an Arizona Biomedical Research Centre New Investigator Award and institutional funds from the University of Arizona that supported this work. The author has no further funding or conflicts of interest to disclose that are relevant to this topic.
Abbreviations
- ERK
- extracellular signal-regulated kinase
- GDP/GTP
- guanosine di/triphosphate
- GPCR
- G protein-coupled receptor
- GRK
- G protein-coupled receptor kinase
- Hsp
- heat shock protein
- MAPK
- mitogen-activated protein kinase
- MEK
- MAPK/ERK kinase
- VEGF
- vascular endothelial growth factor
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics