Abstract
Licorice is a traditional Chinese medicine and recorded to have pain relief effects in national pharmacopoeia, but the mechanisms behind these effects have not been fully explored. Among the hundreds of compounds in licorice, licochalcone A (LCA) and licochalcone B (LCB) are two important components belonging to the chalcone family. In this study, we compared the analgesic effects of these two licochalcones and the molecular mechanisms. LCA and LCB were applied in cultured dorsal root ganglion (DRG) neurons, and the voltage-gated sodium (NaV) currents and action potentials were recorded. The electrophysiological experiments showed that LCA can inhibit NaV currents and dampen excitabilities of DRG neurons, whereas LCB did not show inhibition effect on NaV currents. Because the NaV1.7 channel can modulate Subthreshold membrane potential oscillations in DRG neuron, which can palliate neuropathic pain, HEK293T cells were transfected with NaV1.7 channel and recorded with whole-cell patch clamp. LCA can also inhibit NaV1.7 channels exogenously expressed in HEK293T cells. We further explored the analgesic effects of LCA and LCB on formalin-induced pain animal models. The animal behavior tests revealed that LCA can inhibit the pain responses during phase 1 and phase 2 of formalin test, and LCB can inhibit the pain responses during phase 2. The differences of the effects on NaV currents between LCA and LCB provide us with the basis for developing NaV channel inhibitors, and the novel findings of analgesic effects indicate that licochalcones can be developed into effective analgesic medicines.
SIGNIFICANCE STATEMENT This study found that licochalcone A (LCA) can inhibit voltage-gated sodium (NaV) currents, dampen excitabilities of dorsal root ganglion neurons, and inhibit the NaV1.7 channels exogenously expressed in HEK293T cells. Animal behavior tests showed that LCA can inhibit the pain responses during phase 1 and phase 2 of formalin test, whereas licochalcone B can inhibit the pain responses during phase 2. These findings indicate that licochalcones could be the leading compounds for developing NaV channel inhibitors and effective analgesic medicines.
Introduction
Licorice is a commonly used traditional Chinese medicine consisting of Glycyrrhiza uralensis Fisch., Glycyrrhiza inflata Batalin, and Glycyrrhiza glabra L. according to Chinese national pharmacopoeia (The Committee of Pharmacopoeia of the People’s Republic of China, 1995). The efficacy of licorice decoction includes stomach and spleen protection, pain relief, cough alleviation, and phlegm elimination recoded in ancient Chinse medicine archives (Yang et al., 2015). More beneficial effects of licorice on multiple diseases were found with broader investigations in recent years. It is reported that licorice or its extracts have antiviral and antimicrobial activities (Ahn et al., 2012; Adianti et al., 2014). Recent studies also indicate that licochalcone A (LCA) could relieve the neuropathic pain in chronic constriction injury model and the inflammatory responses induced by lipopolysaccharide (Chu et al., 2012; Li et al., 2021).
The general effects of licorice could be explained by its complex constituents. For now, at least 20 triterpenoids and 300 flavonoids were found in licorice, and each component has specific activities (Yang et al., 2015). This may explain why the effects of licorice are various, but the mechanisms of these effects are little known. With the development of extraction and identification techniques, single molecules in licorice have been isolated, synthesized, and tested under physiologic situations. The molecular mechanisms behind the effects could be unveiled gradually. Among the hundreds of compounds in licorice, LCA is the one that attracted the most attention. The main reports about LCA are related to its antitumor effects (Yang et al., 2015; Hong et al., 2019). We compared the backbone of LCA and another kind of chalcone, loureirin B (LrB), and found that they have high similarities with each other. Since LrB was reported to be a voltage-gated sodium (NaV) channel blocker and have analgesic effects (Chen et al., 2018), we applied LCA in cultured dorsal root ganglion (DRG) neurons and found that it could inhibit NaV currents and dampen excitabilities of DRG neurons. Then we compared the effects of licochalcone B (LCB), which is also a component of licorice and shares the same basic structure with LCA (Yang et al., 2015). We found that LCB did not significantly inhibit the NaV currents in DRG neurons. These differences of the effects on NaV currents between LCA and LCB may provide us with the basis for developing specific NaV channel inhibitors.
NaV channels are reported to consist of 10 different pore-forming α subunits (NaV1.1–9 and NaVX) (Dib-Hajj and Waxman, 2019). Among them, NaV1.7 is mainly expressed in the nociceptors and peripheral neurons, such as DRG neurons, thus Nav1.7 channel has become a potential drug target for pain (Ho and O’Leary, 2011; Li et al., 2018). NaV1.7 channel could modulate subthreshold membrane potential oscillations in DRG neurons, which can palliate neuropathic pain (Li et al., 2018; McDermott et al., 2019). Gain-of-function mutation in NaV1.7 expressing gene SCN9A was related to inherited erythromelalgia (Yang et al., 2004; Fertleman et al., 2006), whereas loss-of-function mutation in SCN9A was linked to channelopathy-associated congenital insensitivity (indifference) to pain and hereditary sensory and autonomic neuropathy type IID (Gingras et al., 2014; Yuan et al., 2013). Inhibition of NaV1.7 might alleviate pain with few side effects because of its exclusive expression in nociceptors, but the number of effective and specific NaV1.7 inhibitors is still rare (Kingwell, 2019). In this research, we found that LCA could block the NaV currents in DRG neurons and the NaV1.7 channels exogenously expressed in HEK293T cells. Further investigations in animal behavior tests showed that LCA could inhibit the pain responses during phase 1 and phase 2 of the formalin test. These are novel findings that revealed the analgesic effects of licochalcones and the molecular mechanisms related to inhibition on voltage-gated sodium channel. This study indicates that licochalcones have the potential to be developed into effective analgesic medicines.
Materials and Methods
Chemicals
Poly-D-lysine (Cat. E607014) and Hanks’ balanced salt solution (HBSS) (Cat. A003210) were ordered from Sangon Co., Ltd (Shanghai, China). Opti-minimal essential medium (Opti-MEM) (Cat. 31985070), Dulbecco’s modified eagle medium (DMEM) (Cat. 11965092), Neurobasal-A (Cat. 10888022), and B-27 supplement (Cat. 17504044) solutions were bought from Gibco (New York, NY), and FBS was ordered from YEASON (Suzhou, China). LCA (Cat. B20409) and ATP-Mg (Cat. T31046) were bought from Yuanye Bio-Technology Co., Ltd (Shanghai, China). The purities of LCA and LCB were high performance liquid chromatography (HPLC) ≥98.0%, and the accuracy of LCA and LCB structures were confirmed by nuclear magnetic resonance technology. LCA and LCB were dissolved in DMSO and diluted with the external solution for electrophysiological experiment, and the same volume of DMSO was added as the vehicle group. Penicillin-streptomycin (Cat. C0230) was bought from Nobleryder (Peking, China), papain (Cat. BS-190) from Biosharp (Hefei, China), and collagenase (Cat. 11179179001) and dispase (Cat. 04942078001) from Roche (Basel, Switzerland). Borosilicate glass (Cat. B15013F) was bought from VitalSense Scientific Instruments Co., Ltd (Wuhan, China) and ExFect2000 Transfection Reagent (Cat. T202-01) from Vazyme (Nanjing, China). Mexiletine (Cat. 5370-01-4) was bought from Aladdin (Shanghai, China) and DMSO (Cat. D2650) from Sigma-Aldrich (St. Louis, MO). Chloral hydrate (Cat. 30037516) and 37% formalin solution (Cat. 33314) were ordered from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Plant Materials
The names of the licorice used in our research have been checked on http://www.theplantlist.org. G. inflata Batalin was bought from Yarkand County Fuyuan Licorice Co., Ltd. (Yarkand, Xinjiang, China). Its flavonoids were extracted according to the methods previously reported (Miyazaki et al., 2020). Roots of G. inflata Batalin were ground into powder, and ethyl acetate was added. The mixture was refluxed at 75°C for 2 hours, and the drug residues were removed. The solvent was dried with nitrogen, and methanol was added for HPLC analysis. Agilent 1100 liquid chromatograph with Agilent SDB C18 chromatographic column (Santa Clara, CA) was used for HPLC analysis. Mobile phase A is 0.5% phosphoric acid aqueous solution, and mobile phase B is acetonitrile. The gradient elution program is as follows: 0→5 minutes, B from 30%→40%; 5→7 minutes, B rose to 50%; 7→30 minutes, B rose to 55%. The detection wavelength was 372 nm, and the flow rate was 1.0 mL/min. The quantities of the licochalcones were measured according to the peak area of the HPLC chromatography. The content of LCA is 2.22% in G. inflata Batalin and 22.20% in its extract. The G. inflata Batalin extract was applied with 0.046 mg/mL mass:volume ratio in electrophysiological experiment to guarantee that the molar concentration of LCA was 30 μM. G. uralensis Fisch. flavonoids were bought from FEIYUBIO Co., Ltd (Cat. FY1246, Nantong, Jiangsu, China). The same mass:volume ratio of G. uralensis Fisch. flavonoids were applied as negative control because it does not contain LCA or LCB according to HPLC analysis. The G. inflata Batalin extract and G. uralensis Fisch. flavonoids were dissolved in DMSO and diluted with the external solution for electrophysiological experiment. The same volume of DMSO was added as the vehicle group. The inhibition rate of NaV current in DRG neuron was calculated as follows:
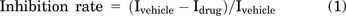
Where Idrug represents the amplitude of NaV current after the drug treatment, and Ivehicle represents the amplitude of NaV current after the vehicle treatment.
Preparation of Dorsal Root Ganglion Neurons
Experiment animals were bought from Wuhan Center for Disease Control and Prevention. All animal experiments were conducted according to the rules of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and strictly following guidelines of the Institutional Animal Care and Use Committees. The Institutional Animal Care and Use Committees checked all protocols, and the animal ethical approval number is 2020-scuec-028. Four-week-old female Kunming mice were anesthetized with isoflurane and sacrificed. The whole spine was dissected out and put in ice-cold HBSS. Extra muscles and tissues were discarded. The spine was cut along the middle line into two symmetrical parts with scissors. Dorsal root ganglions were picked under dissecting microscope. Fibers on DRG were trimmed, and only transparent ganglions were left. The round ganglions were lysised with 2 mg/mL papain in HBSS for 10 minutes at 37°C. During this time, the Eppendorf (Ep) tubes containing ganglions were shaken frequently. The Ep tubes were centrifuged at 4200 rpm for 4 minutes. The supernatant was discarded, and the precipitates were resuspended with 3.75 mg/mL collagenase and 3.75 mg/mL dispase for 10 minutes at 37°C. The Ep tubes were centrifuged at 4200 rpm for 4 minute again. The supernatant was discarded, and 1 mL DMEM was added to stop enzyme digestions. DRG neurons were dissociated by trituration with a fire-polished glass Pasteur pipette (Liu et al., 2009). The cells were centrifuged and resuspended, and seeded on coverslips preincubated with 10 µg/mL poly-D-lysine. Cells were cultured for 2 hours, and extra culture medium (Neurobasal-A with 1× B-27 supplement) was added. After more than 16 hours, DRG neurons were used for electrophysiology recordings.
HEK293T Cell Culture and Transfection
HEK293T cell is a gift from Jing Yao laboratory (School of Life Sciences, Wuhan University, China). The cells were seeded in 24-well plate and cultured in DMEM with 10% FBS and 1% penicillin-streptomycin. When the cell density reached 60%–80%, the cells were transfected with plasmids expressing the human NaV1.7 channel. The transfection procedure was briefly introduced as follows: 800 ng pcDNA3.1-SCN9A (NM_002977.3) plasmid, which expresses NaV1.7, was cotransfected with pIRES2-EGFP plasmid expressing EGFP. Both pcDNA3.1 and pIRES2-EGFP plasmids are gifts from Wenxin Li laboratory (School of Life Sciences, Wuhan University, China). The full-length cDNAs for human NaV1.7 (SCN9A) were subcloned into pcDNA3.1 and sequenced. The HEK293T cells were transfected as the process reported previously (Shi et al., 2021). One Ep tube contained 40 μL Opti-MEM mixed with pcDNA3.1-SCN9A and pIRES2-EGFP plasmids; another one contained 2 μL ExFect2000 in 40 μL Opti-MEM. The two solutions were kept still for 5 minutes without disturbance. After that, they were mixed and left for 20 minutes again. The mixed solution was added gently in the culture medium of HEK293T cells and incubated for more than 36 hours. When the expression of NaV1.7 and EGFP was confirmed with fluorescence microscope, cells were resuspended and seeded on coverslips. After the cells attached on the coverslips, they were picked for electrophysiology recordings.
Electrophysiological Study
Whole-cell patch-clamp recordings were performed using an EPC9 amplifier (HEKA Elektronik, Lambre-cht/Pfalz, Germany) at room temperature (22–24°C). Pipettes pulled from borosilicate glass had resistances of 2–4 MΩ when filled with the internal solution. The internal pipette solution for recording NaV currents contained 130 mM CsCl, 9 mM NaCl, 1 mM MgCl2, 10 mM EGTA, and 10 mM HEPES (pH 7.3 with CsOH) (Chang et al., 2018). The external solution for recording NaV currents contained 131 mM NaCl, 10 mM TEACl, 10 mM CsCl, 3 mM 4-aminopyridine, 2 mM MgCl2, 1 mM CaCl2, 0.3 mM CdCl2, 10 mM HEPES, and 10 mM glucose (pH 7.4 with NaOH) (Chang et al., 2018). Electrophysiology data were analyzed using IGOR (WaveMetrics; Lake Oswego, OR) software. Series resistance was compensated by 80%.
The holding potential was set at −120 mV. Sodium currents (INa) were elicited from −80 mV to +15 mV in 5-mV steps for 50 milliseconds (Zheng et al., 2018). The interval between sweeps lasted for 5 seconds. We evaluated steady-state INa activation by measuring membrane conductance of Na+ (gNa), which was determined with the equation
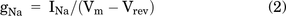 where Vm is the membrane potential, and Vrev is the observed reversal potential for Na+ calculated through current-voltage (I-V) curve. gNa/gNa-max was plotted against membrane potential, and the putative curve was fitted with a sigmoidal function using the Boltzmann model:
where Vm is the membrane potential, and Vrev is the observed reversal potential for Na+ calculated through current-voltage (I-V) curve. gNa/gNa-max was plotted against membrane potential, and the putative curve was fitted with a sigmoidal function using the Boltzmann model:
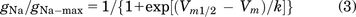
The half-activation voltage was gotten from the fitness curve. For fast inactivation curves, currents were elicited from −130 mV to 0 mV in 10-mV steps for 500 milliseconds, then kept at 0 mV for 20 milliseconds. For slow inactivation curves, currents were elicited from −140 mV to 20 mV in 10-mV steps for 10 seconds, then hyperpolarized to −120mV for 20 milliseconds and depolarized to 0 mV for 20 milliseconds. The currents elicited by depolarization after preconditional voltage steps were analyzed (Goldfarb et al., 2007; Zheng et al., 2018). The half-inactivation voltage was gotten by fitting INa/INa-max against membrane potential with a sigmoidal function using Boltzmann model:
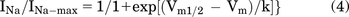
For the current clamp recording on DRG neurons, the internal pipette solution contained 140 mM KCl, 0.5 mM EGTA, 5 mM HEPES, and 3 mM Mg-ATP (pH 7.3 with KOH). The external solution for current clamp recording contained 140 mM NaCl, 3 mM KCl, 2 mM MgCl2, 2 mM CaCl2, and 10 mM HEPES (pH 7.3 with NaOH). Cells with rest membrane potential greater than −50 mV were discarded. Cells were clamped at the resting membrane potential (RP) and elicited with 0–1000 pA ramp current for 500 milliseconds. After the cell state is stable, the action potentials of DRG neurons were recorded before and after drug administration. The number of action potentials, RP, neuronal action potential peak (APP), and action potential threshold were compared before and after the drug treatments. APP was determined by the difference between the APP and the minimum voltage after hyperpolarization, and action threshold was determined by the membrane potential that generated the action potential (Dustrude et al., 2016).
Tetrodotoxin (TTX)-sensitive NaV currents were recorded in large-diameter (>30 μM) DRG neurons. After LCA treatment, the NaV current was washed back to the level before LCA treatment with the external solution perfusion. 300 nM TTX diluted in external solution was applied and see whether TTX could inhibit the Nav currents. The NaV current, which could be completely inhibited by 300 nM TTX, was putative TTX-sensitive NaV current, and the effects of LCA on TTX-resistant NaV currents were tested in small-diameter (<30 μM) DRG neurons. We applied 300 nM TTX in the recording bath and perfusion solution to block the TTX-sensitive NaV currents. The residual NaV currents were putative TTX-resistant NaV currents.
Animal Behavior Test
Animals used for the behavior test were 4-week-old female Kunming mice (20 g ± 2 g), which were commonly used mice strains for formalin-induced pain models (Zhong et al., 2012; Xu et al., 2019). Five to six mice per cage were housed and habituated to the environment for more than 1 week before the experiment. Behavior test was conducted during the daytime, starting at 9 AM in the morning. Mice were put in the observing box and habituated for 20 minutes. LCA or LCB were first diluted in DMSO (25 mg powder in 100 μL DMSO), then diluted in saline to make the final concentration. Mice were injected subcutaneously with different doses of LCA or licochalcone C, including 25 mg/kg, 50 mg/kg, and 100 mg/kg, 30 minutes before the formalin injection. The same volume of saline with diluted DMSO was injected subcutaneously as the vehicle group. The Nav channel blocker (IC50 for Nav1.7 is 1.77 mM) and analgesic drug mexilene were injected with a 50-mg/kg dose in the mice as positive control (Blackburn-Munro et al., 2002; Wu et al., 2013). Thirty minutes later, licochalcone, mexilene, and vehicle groups were injected with a 10-μL 2% formalin solution in the plantar of the right hind paws of the mice. Ten microliters of saline was injected in the plantar of the right hind paw as the saline group to screen out the effects of paw injection. Videos were recorded immediately after the paw injection for more than 30 minutes. The processes were separated into two phases. The first phase is 0–10 minutes, and the second phase is 15–30 minutes (McNamara et al., 2007). The time of mice licking their right hind paws was counted with a stopwatch by two-blinded analyzers.
Statistics Analysis
GraphPad Prism (San Diego, CA) was used to analyze the data. All data were tested for the normality first. Statistical analysis of differences was carried out using Student’s t test or ANOVA combined with Turkey post hoc test if the data were normally distributed. Nonparametric statistical analysis was applied if the data did not distribute normally. P < 0.05 was considered significantly different. Using IGOR (WaveMetrics; Lake Oswego, OR) software, concentration-response curves of LCA were fitted according to the following modified Hill equation:
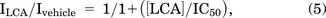 where I represents the amplitude of peak current, and [LCA] represent the concentration of LCA. The IC50 was gotten by four-parametric nonlinear regression analysis constraining bottom to 0 and top to 1.
where I represents the amplitude of peak current, and [LCA] represent the concentration of LCA. The IC50 was gotten by four-parametric nonlinear regression analysis constraining bottom to 0 and top to 1.
Results
LCA Inhibits NaV Current in DRG Neuron
LCA is a kind of chalcone extracted from licorice and shares similar backbone of chalcone structure with LrB (Fig. 1), which has been reported to significantly inhibit NaV current in DRG neuron and has analgesic effects (Chen et al., 2018). We applied LCA to small-diameter (<30 μM) DRG neurons and recorded the NaV currents to see whether LCA had similar effects with LrB on NaV current. Our results showed that 30 μM LCA could significantly inhibit NaV currents in DRG neurons (Fig. 2A). LCB is also a compound in licorice and shares similar backbone structures with LCA and LrB (Fig. 1). We tested the effects of LCB on small-diameter DRG neurons and recorded NaV currents with patch-clamp technology. The voltage clamp results showed that LCB did not inhibit NaV currents in DRG neurons (Fig. 2A).
Chemical structures of licochalcone A (A), licochalcone B (B), and loureirin B (C).
Effects of LCA and LCB on NaV currents in DRG neurons. (A) Representative traces of NaV currents in DRG neurons under vehicle, 30 µM LCA (top) or LCB (middle), and wash treatments; statistics of the inhibition percentage of LCA (n = 7) and LCB (n = 5) compared with vehicle (bottom). (B) Representative traces of NaV currents in DRG neurons with activation stimulus: cells were clamped at −80 mV, and NaV currents were elicited from −60 mV to +10 mV in 10-mV steps for 100 milliseconds. (C) I-V curves of vehicle, LCA treatment, and wash groups. (D) Activation curves of vehicle, LCA, and wash treatments on single cell. (E) Representative traces of NaV currents in DRG neurons with inactivation stimulus: NaV currents were elicited from −60 mV to +10 mV in 10-mV steps for 1000 milliseconds, then kept at −20 mV for 100 milliseconds. (F) Inactivation curves of control, LCA, and wash treatments on single cell. All data are presented as mean ± S.E.M. for n independent observations. Statistical analysis of inhibition percentage was carried out using one-sample t test, and differences between groups was carried out using two-way ANOVA combined with Turkey post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001.
We further recorded and analyzed gating properties of NaV channel under LCA treatment. The maximum activation voltage was significantly higher in the 30-μM LCA treatment group (−12.73 ± 4.28 mV) than in the control (−15.45 ± 4.13 mV) and wash (−13.64 ± 4.72 mV) groups according to I-V curve (Fig. 2, B and C; Supplemental Fig. 1A). The activation and inactivation curves were fitted by single Boltzmann distributions (Griffith et al., 2019). The half-activation voltage was significantly higher in the 30-μM LCA treatment group (−18.21 ± 2.49 mV) than in the control (−25.41 ± 3.18 mV) and wash (−23.30 ± 1.01 mV) groups (Fig. 2D; Supplemental Fig. 1A), and half-inactivation voltage was significantly lower in the LCA group (−51.45 ± 15.75 mV) than in the control (−32.83 ± 7.57 mV) and wash (−43.14 ± 10.21 mV) groups (Fig. 2, E and F; Supplemental Fig. 1A).
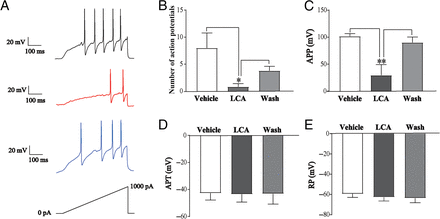
LCA Dampens Excitability of DRG Neuron
NaV channels play a key role in regulating action potentials in DRG neurons (Griffith et al., 2019; McDermott et al., 2019). We used the current clamp to record the changes of action potential before and after LCA treatment and to see whether the inhibition of NaV channels by LCA could affect the DRG neuron excitability. We found that LCA significantly decreased the firing number (control group, 8.00 ± 5.55; LCA-treated group, 0.80 ± 1.16; wash group, 3.80 ± 1.60) and APP (control group, 104.68 ± 6.31 mV; LCA-treated group, 30.53 ± 20.35 mV; wash group, 93.66 ± 10.62 mV) of the DRG neurons (Fig. 3, A−C; Supplemental Fig. 1B). LCA did not significantly change the action potential threshold (control group, −42.75 ± 5.12 mV; LCA-treated group, −43.57 ± 5.77 mV; wash group, −43.21 ± 7.65 mV) or RP (control group, −59.44 ± 9.80 mV; LCA-treated group, −62.80 ± 10.33 mV; wash group, −63.64 ± 12.90 mV) of the DRG neurons (Fig. 3, D and E; Supplemental Fig. 1B). The results of the current clamp indicate that LCA could decrease the frequency and amplitude of the action potentials, thus dampening the excitabilities of DRG neurons.
Effects of LCA on action potentials of DRG neurons. (A) Representative action potential traces of DRG neurons under vehicle (black), LCA treatment (red), and wash (blue). Cells were clamped with rest membrane potentials and elicited with 0–1000 pA ramp current for 500 milliseconds. (B–E) Statistics of the number of action potentials (B), APP (C), action potential threshold (APT) (D), and RP (E) in DRG neurons with LCA treatment. All data are presented as mean ± S.E.M. for n independent observations. Statistical analysis of differences between groups was carried out using one-way ANOVA combined with Turkey post hoc test. *P < 0.05; **P < 0.01.
The Effects of Licorice Extract on NaV Current of DRG Neuron
It has been reported that LCA is a constituent exclusively produced by G. inflata Batalin in Xinjiang (Miyazaki et al., 2020). We used ethyl acetate to extract the flavonoids from the roots of G. inflata Batalin and detected LCA as the main constituents in this species with HPLC analysis (Fig. 4, A−C). It is of interest to see whether the effects of G. inflata Batalin extract are consistent with single-compound LCA. We applied the G. inflata Batalin extract on DRG neurons and recorded the NaV currents. The content of LCA in G. inflata Batalin roots was 2.22% according to the peak area in HPLC chromatography. The concentration of LCA in G. inflata Batalin extract solution was 30 μμ for electrophysiological recordings on DRG neurons. The flavonoids extracted from another species, G. uralensis Fisch., which does not contain LCA, was used as negative control and applied with the same mass-to-volume ratio (0.046 mg/mL) (Fig. 4D). The G. inflata Batalin extract could significantly inhibit NaV currents in DRG neurons, and the inhibition percentage was 77.06 ± 5.92%, whereas the G. uralensis Fisch. extract did not show significant inhibition on NaV currents (Fig. 4, E and F). These results indicate that the effects of G. inflata Batalin extract on NaV currents in DRG neuron were similar with single-compound LCA.
Effects of licorice extract on NaV channels of DRG neurons. (A) HPLC profiles of standard LCA samples; there is a single peak at 12.07 minutes. (A) HPLC profiles of standard LCB samples; there is a single peak at 3.72 minutes. (C) HPLC profiles of ethyl acetate G. inflata Batalin extract; the peak at 11.99 minutes is putative to be LCA, and the content of LCA in G. inflata Batalin is 2.22%. (D) HPLC profiles of G. uralensis Fisch. Flavonoids. (E) Representative traces of NaV currents in DRG neurons under vehicle (black), G. inflata Batalin extract (red), G. uralensis Fisch. flavonoids (cyan), and wash (blue) treatments; the mass:volume ratio of G. inflata Batalin extract used was 0.046 mg/mL to guarantee a 30-μM concentration of LCA. (F) Inhibition percentage of G. uralensis Fisch. and G. inflata Batalin extract on NaV currents of DRG neurons (n = 4); the data are presented as mean ± S.E.M. Statistical analysis of differences between the two groups was carried out using Student’s t test. ***P < 0.001.
LCA Inhibits NaV1.7 Channel Exogenously Expressed in HEK293T Cell
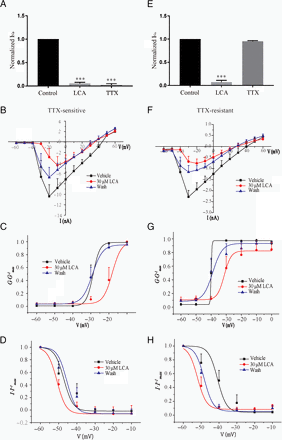
The NaV channels expressed in DRG neurons could be divided into TTX-sensitive and TTX-resistant types (Ho and O’Leary, 2011; Li et al., 2018; Griffith et al., 2019). TTX-sensitive NaV channels are expressed in both large- (>30 μm) and small-diameter (<30 μm) DRG neurons, whereas TTX-resistant NaV channels are dominantly expressed in small-diameter DRG neurons (Ho and O’Leary, 2011; Li et al., 2018; Griffith et al., 2019). To identify which kind of NaV currents were inhibited by LCA, 300 nM TTX diluted with external solution was applied after drug treatment in large-diameter DRG neurons. The Nav currents were recovered to the same level before LCA treatment with external solution perfusion and TTX was applied to see whether the Nav currents could be inhibited by the TTX. The NaV currents which could be completely inhibit by 300 nM TTX was putative TTX-sensitive and analyzed (Fig. 5A). The statistics analysis of I-V curves showed that the maximum activation voltage and the half-activation voltage were significantly higher in the 30-μM LCA treatment group than in the control and wash groups (Fig. 5, B−D; Supplemental Fig. 1C). Then we tested the effects of LCA on TTX-resistant NaV channels in small-diameter DRG neurons. Three hundred nanomolars of TTX was applied in the recording bath and drug perfusion solution. The residual NaV currents were putative TTX-resistant currents (Fig. 5E). The statistical analysis of I-V curves showed that the maximum activation voltage and the half-activation voltage were higher in the 30-µM LCA treatment group than in the control and wash groups (Fig. 5, F−H; Supplemental Fig. 1D). These results indicate that LCA could exert inhibition effects on both TTX-sensitive and TTX-resistant NaV channels.
Effects of LCA on TTX-sensitive and TTX-resistant NaV channels. (A) Statistics of TTX-sensitive NaV currents recorded from large-diameter DRG neurons. (B–D) I-V curve (B), activation curve (C), and inactivation curve (D) of TTX-sensitive NaV currents under vehicle, 30 µM LCA, and wash treatments. (E) Statistics of TTX-resistant NaV currents recorded from small-diameter DRG neurons. (F–H) I-V curve (F), activation curve (G), and inactivation curve (H) of TTX-resistant NaV currents under vehicle, 30 µM LCA, and wash treatments. All data are presented as mean ± S.E.M. for n independent observations. Statistical analysis of differences between groups was carried out using one-way ANOVA combined with Turkey post hoc test. ***P < 0.001.
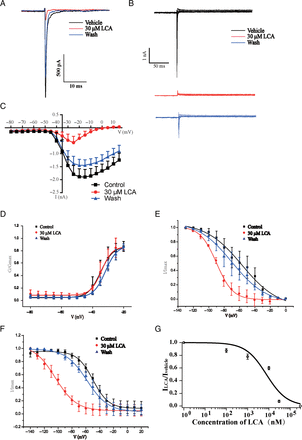
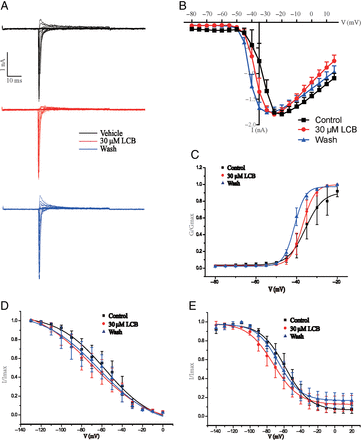
According to the above results, the large-diameter DRG neurons exclusively expressing TTX-sensitive channels could be blocked by LCA. We expressed one subunit of TTX-sensitive NaV channel—NaV1.7—in HEK293T cells to observe the effects of LCA. Our results showed that LCA could both inhibit NaV1.7 currents and shift the I-V curve to depolarization direction (Fig. 6, A−C). Analysis on voltage-gating properties indicated that LCA did not significantly increase the half-activation voltage of NaV1.7 channel (Fig. 6D; Supplemental Fig. 1E), whereas LCA significantly decreased the fast and slow half-inactivation voltage of NaV1.7 channel (Fig. 6, E and F; Supplemental Fig. 1E). To get the IC50 value of LCA on NaV1.7, we treated the NaV1.7-expressing HEK293T cells with different concentrations of LCA for 1.5 minutes. The IC50 value (7.6 ± 3.5 μM; n = 5) was calculated by fitting concentration-response curve to Hill equation (Fig. 6G). We also applied LCB on HEK293T cells expressing NaV1.7 and analyzed the voltage-gating properties. The half-activation and inactivation voltage of NaV1.7 channel were not significantly changed by LCB (Fig. 7, A−E).
Effects of LCA on NaV1.7 channels exogenously expressed on HEK293T cells. (A) Representative traces of NaV1.7 currents in HEK293T cells before or after LCA treatment. (B) Representative traces of NaV currents in DRG neurons with activation stimulus: cells were clamped at −120 mV, and NaV currents were elicited from −80 mV to 15 mV in 5-mV steps for 50 milliseconds. (C) I-V curves of NaV1.7 channel with LCA treatment; the data are presented as mean ± S.E.M. for n independent observations, and statistical analysis of differences between groups was carried out using two-way ANOVA combined with Turkey post hoc test. (D–F) Activation curves (D), fast inactivation curves (E), and slow inactivation curves (F) with LCA treatment. (G) Concentration- response curve of NaV1.7 current with LCA treatment; y-axis represents the ratios of NaV current amplitudes (ILCA/Ivehicle), and x-axis represents the concentration of LCA. The curve was fitted by Hill equation.
Effects of LCB on NaV1.7 channels exogenously expressed on HEK293T cells. (A) Representative traces of NaV currents in HEK293T cells with activation stimulus: cells were clamped at −120 mV, and NaV currents were elicited from −80 mV to 15 mV in 5-mV steps for 50 milliseconds. (B) I-V curves of NaV1.7 channel with LCA treatment; the data are presented as mean ± S.E.M. for n independent observations, and statistical analysis of differences between groups was carried out using two-way ANOVA combined with Turkey post hoc test. (C–E) Activation curves (C), fast inactivation curves (D), and slow inactivation curves (E) with LCB treatment.
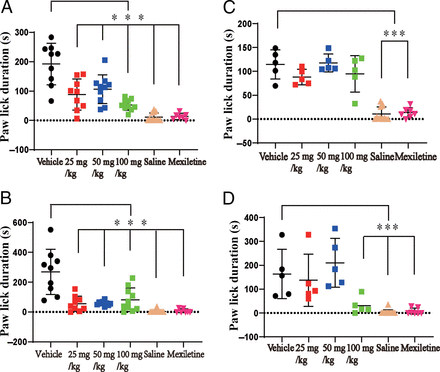
The Effects of LCA and LCB on Formalin-Induced Pain Responses
NaV1.7 has been reported to be predominantly expressed in nociceptors and closely related to pain in humans and animals (Kingwell, 2019; McDermott et al., 2019). Since LCA had significant inhibition effects on NaV currents in DRG neurons and NaV1.7 channels exogenously expressed in HEK293T cells, it might also have pain-relief effects. We used a pain behavior model - formalin test to investigate the effects of LCA on acute pain responses of animals (Uniyal et al., 2021). There are two phases in formalin tests: the first phase was mainly conducted by neuropathic pain, whereas the second phase was related to the activation of complex downstream pathways (Fernandes et al., 2018; Uniyal et al., 2021). Our results showed that different concentrations (25 mg/kg, 50 mg/kg, and 100 mg/kg) of LCA could alleviate the pain responses in both the first and the second phases of the formalin test (Fig. 8, A and B). We applied 50 mg/kg mexiletine, which is a known NaV channel blocker and analgesic drug, as the positive control during the tests (Blackburn-Munro et al., 2002). The results of mexiletine showed that it could significantly decrease the licking time of mice after formalin injection in their right hind paws during both phases, which confirmed that our animal models are successful. The effects of LCA were consistent with their effects on NaV channels in DRG neurons and NaV1.7 expressed in HEK293T cells. LCB did not affect the responses during the first phase of formalin test but could inhibit the licking time during the second phase in a relatively high concentration (100 mg/kg) (Fig. 8, C and D). These results indicate that LCB behaves differently with LCA on NaV channels and neuropathic pain but may exert effects on molecular signaling pathways during the second phase of formalin test.
Effects of LCA and LCB on formalin-induced pain responses. (A) Statistics of paw-licking time during the first phase of formalin test under vehicle (n = 9), LCA in different concentrations (n = 9 for 25 mg/kg, n = 10 for 50 mg/kg, and n = 10 for 100 mg/kg), saline (n = 12), and Mexiletine (n = 7) treatments. (B) Statistics of paw-licking time during the second phase of formalin test under vehicle, LCA in different concentrations, saline, and mexiletine treatments. (C) Statistics of paw-licking time during the first phase of formalin test under vehicle (n = 5), LCB in different concentrations (n = 5 for 25 mg/kg, n = 5 for 50 mg/kg, and n = 5 for 100 mg/kg), saline (n = 12), and mexiletine (n = 7) treatments. (D) Statistics of paw-licking time during the second phase of formalin test under vehicle, LCA in different concentrations, saline, and mexiletine treatments. All data are presented as mean ± S.E.M. for n independent observations. Statistical analysis of differences between groups was carried out using two-way ANOVA combined with Turkey post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the vehicle group.
Discussion
As a traditional Chinese medicine, licorice is commonly applied to protect the stomach and spleen, alleviate pain, and improve the symptoms of cough and phlegm (Yang et al., 2015). Researchers recently discovered that LCA could relieve neuropathic pain and inflammatory responses (Chu et al., 2012; Li et al., 2021). Although licorice was proved to have effects on multiple diseases, the molecular mechanisms have rarely been disclosed. LCA and LCB are chalcones in licorice and share the same backbones with each other. The results of electrophysiological recording showed that LCA could inhibit NaV currents in DRG neurons. These results indicate that LCA has the potential to be developed into NaV inhibitors. Although LCA could significantly inhibit NaV currents in mouse DRG cells, LCB did not block the NaV currents. LCB also did not have effects on activation or inactivation of NaV1.7 channel. Structural analysis showed that the predicted physicochemical properties of LCA and LCB, such as LogP and pKa, are different (data not shown). This might be because LCA has olefin substituents consisting of five carbon atoms on the aromatic B ring, whereas LCB has no such side chain but one extra hydroxyl group, which suggests that the olefin substituent on the aromatic B ring of licochalcone may play a key role in blocking the NaV channel. The discrepancy of effects between LCA and LCB may guide us to discover the key chemical groups to inhibit the specific channels and develop more effective NaV channel inhibitors.
NaV channels in DRG neurons could modulate the membrane potential oscillations and transduce sensories, like pain and itch (Li et al., 2018; Griffith et al., 2019). We used current clamp to record the action potentials of DRG neurons to see whether inhibition of NaV channels by LCA could dampen the excitabilities of these neurons. The results showed that LCA could significantly decrease the firing frequencies and amplitudes of DRG neurons. We further conducted formalin test and observed that LCA had analgesic effects in these animal models. It has been confirmed that pain responses during phase 1 of formalin test is neuropathic pain, which is mediated by direct nociceptor neurotransmission, whereas phase2 involves more complex signaling pathways, such as immune cell infiltration and the activation of glia (Fernandes et al., 2018; Uniyal et al., 2021). Our results showed that LCA could decrease the licking time of mice during both phase 1 and phase 2 of formalin test. These results were consistent with changes of NaV currents in DRG neurons and inhibition of NaV1.7 exogenously expressed in HEK293T cells. Previous studies have reported that NaV1.7 is widely expressed in DRG neurons and is devoted both to neuropathic pain and inflammatory pain (Nassar et al., 2004; Dib-Hajj et al., 2013). Inhibition of NaV1.7 by LCA may lead to alleviation of pain responses during both phase 1 and phase 2 in formalin test. Although LCB did not show inhibitory effects on DRG NaV currents, it could decrease the licking time during phase 2 of formalin test. We suspect that LCB may be able to inhibit other channels or signaling molecules that were involved in the pain or inflammatory responses during phase 2. It is of great interest to explore the mechanisms of this phenomenon.
DRG neurons could be divided into diverse groups according to their size, function, and molecular characteristics (Ho and O’Leary, 2011; Dib-Hajj et al., 2013). Small-size (<30 μm) DRG neurons express both TTX-sensitive NaV channels, such as NaV1.7, and TTX-resistant NaV channels, such as NaV1.8 and NaV1.9 (Ho and O’Leary, 2011; Griffith et al., 2019). Large-size (>30 μm) DRG neurons mainly express TTX-sensitive NaV channels, such as NaV1.1, NaV1.6, and NaV1.7 (Ho and O’Leary, 2011; Griffith et al., 2019). Three hundred nanomolars of TTX was applied after LCA administration and wash-back in large-size DRG neurons to confirm that the recorded NaV currents could be completely blocked. The results indicated that LCA could inhibit TTX-sensitive NaV channels in large-size DRG neurons. Although we observed the inhibition on Nav1.7 in HEK293T cells, the effects of LCA on other subunits, such as Nav1.1 and Nav1.6, need to be explored. To separate the TTX-resistant NaV currents, 300 nM TTX was applied in the bath solution ,and the residual NaV currents in small-size DRG neurons were recorded. Similar to the results in large-size DRG neurons, LCA could inhibit TTX-resistant NaV currents in these cells. These results indicate that LCA may be able to inhibit TTX-resistant NaV channels, like NaV1.8 and NaV1.9. We need further express other types of NaV channel subunits in HEK293T cells to observe the effects of LCA in future.
Authorship Contributions
Participated in research design: Yin.
Conducted experiments: Zhao, Zhang, Long, Wang, Yu, Zhou, Li, Xue, Hu.
Performed data analysis: Zhao, Zhang, Long, Wang, Yin.
Wrote or contributed to the writing of the manuscript: Zhao, Yin.
Footnotes
- Received November 27, 2022.
- Accepted June 8, 2023.
This work is supported partly by grants from the National Natural Sciences Foundation of China [81373379 and 81641186] and the National Key R and D Program of China [2019YFC1712402] (to S.Y.); the National Natural Sciences Foundation of China [32000685] and Natural Sciences Foundation of Hubei Province [2020CFB348] (to Q.Z.); the Fundamental Research Funds for the Central Universities, South-Central Minzu University [CZZ19005] (to S.Y.) and [CZQ23026] (to Q.Z.); and Knowledge Innovation Program of Wuhan-Shuguang Project [2022020801020412] (to Q.Z.).
All authors declare no interest conflicts.
↵1Q.Z., X.Z., and S.L. contributed equally to this work.
↵
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
Abbreviations
- APP
- action potential peak
- DMEM
- Dulbecco’s modified eagle medium
- DRG
- dorsal root ganglion
- Ep
- Eppendorf
- gNa
- membrane conductance of Na+
- HBSS
- Hank’s balanced salt solution
- HPLC
- high performance liquid chromatography
- I
- amplitude of peak current
- I-V curve
- current-voltage curve
- INa
- sodium current
- Ivehicle
- amplitude of NaV current after the vehicle treatment
- LCA
- Licochalcone A
- LCB
- Licochalcone B
- LrB
- loureirin B
- NaV
- voltage-gated sodium
- Opti-MEM
- opti-minimal essential medium
- RP
- resting membrane potential
- TTX
- tetrodotoxin
- Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics