Abstract
The nuclear receptor constitutive active receptor (CAR) translocates into liver nuclei after phenobarbital (PB) treatment, and activates the conserved enhancer called the PB-response element module (PBREM) found in CYP2B genes. We have examined whether CAR regulates the dimorphic induction by PB of the CYP2B1 gene in Wistar Kyoto (WKY) rats. Northern blot analysis showed that PB induced CYP2B1 mRNA in male WKY rats but not female rats. An in situ injected PBREM-luciferase reporter gene was activated by PB only in the male livers. Western blot analysis revealed extremely low levels of CAR in the cytosols of female livers compared with male counterparts. CAR was accumulated in the liver nucleus of male rats in response to PB treatment, whereas the receptor was barely detectable in the liver nuclei of PB-induced females. These sexually dimorphic responses of PBREM and CAR to PB treatment were not observed with Fisher 344 rats, in which CYP2B1 mRNA was induced in both sexes. Thus, these results indicate that CAR is a regulatory factor that leads to the sexual dimorphic induction of CYP2B1 gene in WKY rats.
Liver microsomal cytochrome P450s (P450s) catalyze metabolic detoxification of xenochemicals, such as pharmaceutical drugs and both man-made and naturally occurring chemicals in the environment. Liver P450 genes can be induced by exposure to xenochemicals, resulting in an increase in the organ's metabolic capability (Conney, 1982). It is known that this xenochemical inducibility of P450 genes is often sexually dimorphic in rats and mice. Consequently, P450 induction may lead to sex-dependent susceptibility to toxic and/or carcinogenic xenochemicals. Phenobarbital (PB) is one such chemical that inducesCYP2B genes in males but not females of certain rodent strains under some conditions (Waxman et al., 1985; Yamazoe et al., 1987; Corcos, 1992; Honkakoski et al., 1992; Waxman and Azaroff, 1992). The mechanism regulating the sex-dependent induction ofCYP2B genes remains unknown.
A conserved PB-responsive enhancer has recently been defined in mouse, rat, and human CYP2B genes (Trottier et al., 1995;Honkakoski and Negishi 1997; Honkakoski et al., 1998a; Honkakoski and Negishi, 1998; Stoltz et al., 1998; Sueyoshi et al., 1999). The PB-responsive enhancer module (PBREM) found in the mouse and human genes constitutes a 51-bp DNA sequence containing two nuclear receptor binding DR-4 motifs, NR1 and NR2 (Honkakoski et al., 1998a; Sueyoshi et al., 1999). Using DNA affinity purification combined with Western blot analysis, we identified the liver-enriched nuclear receptor constitutive active receptor (CAR) as a PB-responsivetrans-activator of PBREM (Honkakoski et al., 1998b). CAR, as a heterodimer with retinoid X receptor (RXR), increases its binding to NR1 in response to PB treatment in liver, resulting in the induction ofCYP2B genes. CAR seems to play a central role in the induction of CYP2B genes, not only by PB but also by other PB-type compounds, such as 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene, chlorpromazine, 1,1,1-trichloro-1,2-bis(o,p′-chlorophenyl)ethane, and 2,3,3′,4-tetrachlorobiphenyl (Honkakoski et al., 1998a; Sueyoshi et al., 1999). Although CAR spontaneously translocates into nuclei of transfected HepG2 cells, it seems to be a cytoplasmic receptor in the livers of noninduced mice and to translocate to the nuclei after PB treatment (Kawamoto et al., 1999). Thus, the nuclear translocation of CAR is an essential step occurring at an early stage of PB induction ofCYP2B genes.
Jefcoate and his colleagues (Larsen et al., 1994; Larsen and Jefcoate, 1995; Ikegwuonu et al., 1996; Ganem and Jefcoate, 1998) reported a series of studies regarding sex-dependent induction by PB of CYP2B and other xenochemical-metabolizing enzymes in rat livers. PB induces CYP2B proteins only in male Wistar Kyoto (WKY) rats, whereas it induces CYP2B proteins in both male and female Fischer 344 (F344) rats. In this study, we have investigated, using the WKY-F344 rat model, the mechanism by which CAR may regulate the male-specific P450 induction in WKY rats. Performing Northern blot analyses of CYP2B1 mRNA, in situ transfection assays using a PBREM-thymidine kinase (tk) promoter-luciferase (Luc) plasmid, and Western blot using anti-human CAR antibodies, we herein present experimental considerations that lead us to reiterate the critical roles played by CAR and PBREM in regulating the PB-inducible CYP2B genes.
Experimental Procedures
Materials.
[γ-32P]ATP (>6000 Ci/mmol) and [α-32P]dCTP (>6000 Ci/mmol) were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). pRL-CMV and pGL3 plasmids were from Promega (Madison, WI). Oligonucleotides were synthesized with an ABI392 DNA/RNA synthesizer obtained from Life Technologies (Grand Island, NY). Anti-RXRα was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Two antisera against CAR were produced by immunizing rabbits with a peptide CALFSPDRPGVTQREEIDQLQE (anti-CAR peptide antibodies) or a bacterially expressed recombinant protein, human CAR fused to glutathioneS-transferase (anti-human CAR antibodies), and these antisera were purified using the peptide or purified glutathioneS-transferase-mouse CAR fusion protein as the affinity resins.
Animals.
WKY and F344 rats (5–7 weeks) were obtained from Charles River Laboratories (Raleigh, NC). PB (Sigma, St. Louis, MO) was injected intraperitoneally in water at a dose of 100 mg/kg of body weight.
DNA Cloning.
Genomic DNA was isolated from WKY and F344 rat livers using TRIZOL reagent (Life Technologies), from which an up-stream region containing the PBREM sequence of the CYP2B1gene was amplified using the oligonucleotides 5′-GATCGTGGACACAACC-3′ and 5′-CACTTCCTGCATGGAATG-3′. Amplified DNA was subcloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA) and subsequently sequenced to verify the sequence of PBREM.
RT-PCR was employed to amplify CAR cDNA from liver RNAs prepared from WKY and F344 rats. SuperScript Preamplification System (Life Technologies) was used to synthesize first strand cDNAs using the following primers designed from human and mouse CAR cDNA sequences (Baes et al., 1994; Choi et al., 1997) and a rat expressed sequence tag clone sequence (accession number H34574): 5′-TGCTGCCTAAGRGAARCAGGAG-3′ (R = A or G) and 5′-CTGGGAAAGGATCCAAGCCTGGG-3′. The amplified cDNAs were subcloned into pCR2.1-TOPO and sequenced at both strands.
Plasmids.
tk-Luc was previously constructed using a pGL3 vector (Honkakoski et al., 1998a; Sueyoshi et al., 1999). PBREM-tk-Luc containing the PBREM found in the CYP2B1 gene, a tk promoter and a Luc gene, and (NR1)3-tk-Luc, consisting of three copies of the rat NR1 sequence, a tk promoter, and a Luc gene, were constructed. Four oligonucleotides rPBREM-1, 5′-CTAGCTCTGTACTTTCCTGACCTTGGCACAGTGCCACCATCAACTTGACTGACACCA-3′, rPBREM-2, 5′-GATCTGGTGTCAGTCAAGTTGATGGTGGCACTGTGCCAAGGTCAGGA A AGTACAGAG-3′, 3xrNR1–1, 5′-CTAGCTCTGTCATTTCCTGACCTTGGGATCCTCTGTCATTTCCTGACCTTGGTCGACTCTGTCATTTCCTGACCTTGA-3′ and 3xrNR1–2, 5′-GATCTCAAGGTCAGGAAAGTACAGAGTCGACCAAGGTCAGGAAAGTACAGAGGATCCCAAGGTCAGGAAAGTACAGAG-3′ were synthesized. rPBREM-1 and rPBREM-2, or 3xrNR1–1 and 3xrNR1–2, were annealed and subcloned into NheI and BglII sites of the tk-Luc vector. The sequences of the plasmids constructed were confirmed by sequencing. DNA was purified with a QIAfilter plasmid Giga kit (Qiagen, Valencia, CA) and dissolved in Dulbecco's modified Eagle's medium (Sigma).
Northern Blot.
Total RNA from pools of three to five untreated and PB-treated rat livers was isolated with TRIZOL reagents. Twenty micrograms of RNA was subjected to 1% agarose-formaldehyde gel electrophoresis, transferred to Hybond-N+membrane (Amersham Pharmacia Biotech), and hybridized with32P-labeled probes. For detection of CYP2B1 and CYP2B2 mRNA, synthesized oligonucleotides described previously were used (Giachelli and Omiecinski, 1986). To verify the amount of RNA loaded, the membrane was rehybridized with mouse β-albumin cDNA. The hybridized membrane was exposed to X-OMAT AR film (Kodak, Rochester, NY).
In Situ DNA Injection.
In situ injection assays were performed as described by Park et al. (1996) with minor modifications. Briefly, rats were treated with dexamethasone (Sigma) subcutaneously at 1 mg/kg. Twenty-four hours later, a portion of the liver was exposed through a ventral midline incision after anesthetization with ether, and reporter plasmids (250 μg/rat) tk-Luc, PBREM-tk-Luc or (NR1)3-tk-Luc, and a control plasmid pRL-CMV (50 μg/rat) were injected. The rats were treated with dexamethasone subcutaneously and with saline or PB (100 mg/kg) intraperitoneally 10 min and 4 h after the surgery, respectively. Twenty hours after the injection of saline or PB, the rats were sacrificed and the reporter activities in liver extracts were measured as follows. One gram of the liver was homogenized in 4 ml of passive lysate buffer (Promega) and the homogenate was centrifuged at 12,000g for 20 min at 4°C. Resultant supernatants were subjected to the Dual-Luciferase reporter assay (Promega). Reporter activities were normalized using activities of the coinjected pRL-CMV as a control.
Western Blot.
Preparation of nuclear extracts from rat livers and DNA-affinity chromatography were carried out as described previously (Honkakoski et al., 1998b). Rat livers were homogenized in 3 volumes of 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 150 mM sodium chloride, 20 mM sodium molybdate, 1% Triton X-100, 0.5% Nonidet P-40, 0.2 mM sodium ortho-vanadate, 0.2 mM phenylmethylsulfonyl fluoride, 2 μg/ml pepstatin A, and 2 μg/ml leupeptin. This homogenate was centrifuged at 10,000g for 30 min at 4°C and the resulting supernatant was used as total liver extracts. For cytosolic fractions, rat livers were homogenated in 3 volumes of 50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 15% glycerol, 1 mM dithiothreithol, 0.2 mM phenylmethylsulfonyl fluoride, 2 μg/ml pepstatin A, and 2 μg/ml leupeptin, and centrifuged at 105,000g for 1 h at 4°C. The resulting supernatant was used as cytosol. For Western blot analysis, 12 μg of total nuclear extracts, NR1-affinity purified proteins from 250 μg of total nuclear extracts, or 100 μg of total liver extracts or cytosols were separated on 10% SDS-polyacrylamide gel and transferred to Immobilon-P (Millipore, Bedford, MA). The membrane was incubated with rabbit anti-CAR peptide antibodies, anti-human CAR antibodies or anti-RXRα antibodies. Then, immunoreactive bands were visualized with enhanced chemiluminescence Western blotting detection reagents (Amersham Pharmacia Biotech). Rat CAR cDNA subcloned into pCR3 (Invitrogen) was expressed by in vitro transcription/translation system with TNT T7 Coupled Reticulocyte Lysate System (Promega), and a small portion of the protein expressed and nuclear extracts prepared from female mouse livers were also subjected to Western blot analysis.
RT-PCR.
Levels of rat CAR mRNA were measured by RT-PCR with Advantage 2 PCR kit (CLONTECH, Palo Alto, CA), according to the user manual, using the oligonucleotides 5′-TCTCACTCAACACTACGTTC-3′ and 5′-CTGGGAAAGGATCCAAGCCTGGG-3′. The amplification cycles used were as follows: first denaturing at 94°C for 2 min and 32 cycles of 30 s each of 94°C, 57°C, and 72°C reactions. The aliquots of the reactions were electrophoresed on an agarose gel and visualized with ethidium bromide. Rat CAR cDNA subcloned into pGEM-3Z plasmid (Promega) was used for a positive control of the experiments. The PCR products were subcloned and sequenced. β-Actin mRNA was also amplified for the control.
Gel Shift Assay.
Nuclear extracts were incubated with 1 μg of normal rabbit IgG (Pierce, Rockford, IL) or anti-human CAR IgG for 10 min at room temperature. The incubation mixture was subjected to gel shift assays using 32P-labeled NR1 probe as described previously (Honkakoski et al., 1998b). For a control, in vitro translated rat CAR and human RXRα were used for the gel-shift assay.
Results
Sex-Dependent Increase of CYP2B1 mRNA.
Northern blot analysis was performed to examine whether sex-dependent induction by PB of CYP2B1 occurred at the mRNA level in rat liver. Whereas mRNA was greatly increased in a time-dependent manner in PB-treated male WKY rats, its level remained largely unchanged in PB-treated female WKY rats (Fig. 1). Contrary to this sex-dependent induction, both sexes of F344 rats exhibited dramatic increases in CYP2B1 mRNA after PB treatment (Fig. 1). CYP2B2 mRNA displayed the similar sexual dimorphisms in these rats (data not shown). The levels of β-albumin mRNA were not affected by PB treatment in either sex of WKY or F344 rats (Fig. 1). Characteristically, female WKY rats responded poorly to PB, with little increase of CYP2B1 mRNA, consistent with a previous finding that CYP2B protein was not induced by PB in these female rats (Larsen et al., 1994).
Induction by PB of CYP2B1 mRNA in WKY and F344 rats. Total RNAs were prepared from rat livers at various time points after PB treatment and subjected to Northern blot analysis as described underExperimental Procedures. Numbers indicate hours after PB treatment.
Sequences of Rat CAR and PBREM.
To eliminate the possibility that any sequence differences in CAR and/or PBREM cause the sexual dimorphic induction, the following procedures were undertaken. First, CAR cDNAs were cloned from livers of female and male WKY and F344 rats (GenBank accession numbers are AF133095 and AF133094, respectively). Deduced amino acid sequences revealed that all rat CARs contain 358 amino acid residues with identical sequences (Fig.2A). Rat CAR shared 91% and 77% amino acid sequence identities with mouse and human counterparts, respectively. We then prepared genomic DNAs from WKY and F344 rats and cloned a CYP2B1 gene's 5′-flanking region that contained the PBREM-corresponding sequence. The 51-bp sequence of PBREM from these rat strains was identical, which is aligned with a center portion of PB response unit (PBRU) previously defined to the CYP2B2gene (Fig. 2B) (Trottier et al., 1995; Kim and Kemper, 1997; Stoltz et al., 1998). The PBREM found in the CYP2B1 gene differs by a single nucleotide from the mouse PBREM of the Cyp2b10gene. Nevertheless, there was neither a strain- nor a sex-dependent sequence difference of CAR or PBREM in these rats.
Sequences of rat CAR and PBREM. A, deduced amino acid sequence of rat CAR (GenBank accession numbers AF133094 and AF133095) is compared with the mouse and human counterparts (Baes et al., 1994;Choi et al., 1997). B, PBREM sequence of the CYP2B1 gene used for our present study is aligned with the PB-response enhancer sequences reported previously: rat PBRU of the CYP2B2gene and mouse PBREM of the Cyp2b10 gene. The PBRU(1) is the sequence reported by Anderson's group (Trottier et al., 1995;Stoltz et al., 1998). Underscores indicate two critical elements for PBRU activity: a putative glucocorticoid receptor binding site on 5′-end and an accessory element called AF1 on 3′-end. The PBRU(2) defines the sequence based on an in vivo footprinting assay by Kemper's group (Kim and Kemper, 1997), in which a dotted underline was protected in a native chromatin from noninduced rat livers. The mouse PBREM sequence is taken from our previous reports (Honkakoski and Negishi, 1997; Honkakoski et al., 1998a). Two nuclear receptor binding motifs NR1 and NR2, and an NF1 site are boxed.
Sex-Dependent Activation of PBREM.
We examined whether PBREM was activated differently in PB-treated male and female WKY rats. For this, PBREM-tk-Luc plasmid was in situ injected into the livers of WKY rats, followed by PB treatment. As a result, the reporter activity (i.e., PBREM enhancer activity) was enhanced by approximately 6-fold in male WKY rats after PB treatment, whereas it was hardly activated in PB-treated female WKY rats (Fig. 3). Activation of PBREM by PB seemed to be sexually specific to male WKY rats. In sharp contrast, PBREM was activated by PB treatment 3.5- to 4.5-fold in both sexes of F344 rats, although the activation in male rats was slightly more effective than in female rats (Fig. 3). These rates of increase of induction in F344 rats were similar to those observed previously using the 179-bp PB responsive element (now called PBRU) by Park et al. (1996). Thus, the failure of PB to activate PBREM clearly correlated with the lack of an increase of CYP2B1 mRNA in PB-treated female WKY rats.
Enhancer activity of an in situ injected PBREM and NR1 into rat livers. Either a tk-Luc, PBREM-tk-Luc, or (NR1)3-tk-Luc construct was directly injected into WKY and F344 rat livers and the reporter activity in the liver extract was measured as described under Experimental Procedures. Fold of induction represents ratio of the activity in PB-treated rat livers to that in the saline-treated animals. Four or five injections were carried out for each group. Open, closed, and hatched bars indicate activities of tk-Luc, PBREM-tk-Luc, and (NR1)3-tk-Luc reporters, respectively.
It is known that NR1 alone is capable of being activated in HepG2 cells and of responding to PB in mouse primary hepatocytes (Sueyoshi et al., 1999). Therefore, (NR1)3-tk-Luc plasmid was used for in situ transfection assays to determine whether NR1 also displayed sex-dependent activation in PB-treated WKY rats. Basal NR1 activity was approximately 2-fold higher in nontreated male WKY rats, compared with the corresponding basal activity of PBREM (Fig. 3). Despite its higher basal activity, NR1 activity was enhanced 4.1-fold in response to PB in the male rats (Fig. 3). Basal activity of NR1 was at a 5-fold higher level than that of PBREM in nontreated female WKY rats and was not at all enhanced by PB treatment. Thus, the male-specific PB responsiveness was also seen at the level of NR1 activity. Unexpectedly, PB did not activate NR1 in any of the F344 rats (Fig. 3). Again, the higher basal activities of NR1 seemed to overwhelm the ability of NR1 to respond to PB induction in F344 rats. Thus, PBREM required the entire sequence to properly respond to PB in regulating the sex-dimorphic transcription of the CYP2B1 gene in rats.
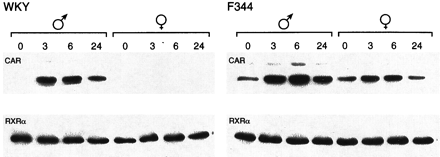
Sexually Dimorphic Expression of CAR.
CAR is translocated into the nucleus after PB treatment in mouse livers and primary hepatocytes; this nuclear translocation is an initial step occurring during PB-induced trans-activation of PBREM (Kawamoto et al., 1999). The poor activation by PB of PBREM as well as NR1 in female WKY rats implied that CAR might not be present in the nucleus of these female rats. To examine this, Western blot analysis was employed on NR1-affinity purified liver nuclear extracts prepared from WKY and F344 rats at various time points after PB treatment, using anti-CAR antibodies. Although binding of CAR to NR1 was hardly detected in liver nuclear extracts from nontreated male WKY rats, it was dramatically increased after PB treatment in a time-dependent manner (Fig.4). In sharp contrast, CAR was at an undetectable level in the nucleus of either nontreated or PB-treated female WKY rats (Fig. 4). No such strong sexual dimorphisms in the nuclear CAR were observed in F344 rats, although the liver nuclei of the male rats contained more CAR proteins than those of the female rats (Fig. 4). The typical nuclear protein RXRα remained at similar levels before and after PB treatment in WKY and F344 rats of both sexes, serving as an internal control for the preparation of nuclear extracts (Fig. 4). Thus, CAR seemed to be a limiting factor for the PB responsiveness in female WKY rats. An apparent lack of CAR in the nuclei of female WKY rats could mean that either CAR is not expressed or is unable to undergo nuclear translocation. To examine these possibilities, we first performed gel shift assay using the nuclear extracts and NR1 probe (Fig. 5). The binding of NR1 with nuclear protein was increased after PB treatment in male WKY rats and the preincubation with the anti-human CAR antibody effectively inhibited the binding. On the other hand, PB treatment did not change greatly the NR1 binding with the female nuclear extracts. Moreover, the antibody did not specifically affect this binding. These results suggested that PB treatment increased a CAR-RXRα heterodimer in the nucleus of male, but not female, WKY rats. The antibody-insensitive binding of NR1 with the female nuclear extracts implied the presence of another protein that can bind to the NR1 site in the female rats.
The receptor CAR in liver nuclear extracts from PB-treated rats. Both preparation of liver nuclear extracts and NR1-affinity purification of CAR were carried out as described underExperimental Procedures. For Western blots, anti-CAR peptide antibodies were used for affinity-purified fractions (top). In addition, total nuclear extracts were immunostained using anti-RXRα antibodies (bottom). Numbers indicate hours after PB treatment.
Changes in NR1 binding of WKY rat nuclear extracts by PB treatment. Nuclear extracts were prepared from control (lanes C) and PB-treated (lanes PB) male and female WKY rat livers. The extracts (1.5 μg) or mixture of rat CAR and human RXRα translated in vitro (lanes rCAR + hRXRα) were subjected to gel shift assays. The proteins were incubated with 1 μg of normal rabbit IgG (−) or anti-human CAR IgG (+), then mixed with 32P-labeled NR1.
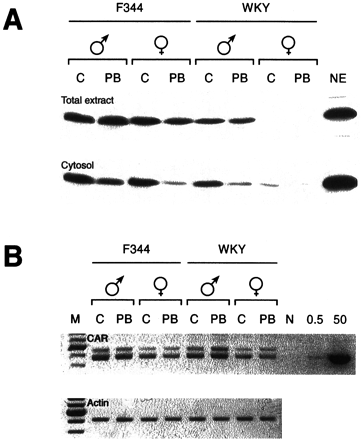
Next, total liver extracts and cytosols were subjected to Western blot analysis using anti-human CAR antibody. The sex differences in the levels of CAR were already observed with the total liver extracts as well as cytosols (Fig. 6A). Consistent with its level in the nucleus, CAR was detected at high levels in the livers of F344 male and female rats and in those of male WKY rats. Female WKY rats, on the other hand, showed a barely detectable level of CAR in the total liver extracts. These different levels of CAR in the total liver extracts reflected those in the liver cytosols. Consistent with the fact that CAR in the cytoplasm translocates to the nucleus after PB treatment, the levels of CAR were decreased in the cytosols prepared from the PB-treated rats (Fig. 6A). CAR was nearly absent in the liver cytosol of female WKY rats, resulting in the near absence of this receptor in the nucleus. RT-PCR was then performed to measure the levels of CAR mRNA in rat livers (Fig. 6B). Under the conditions used, the two amplified DNAs were produced and separated on an agarose gel: the lower band represented the mature CAR mRNA, and the upper band contained one of splice variants of CAR mRNA. The mRNA remained constant level during PB treatment. No strong sex- or strain-dependent differences were observed in the CAR mRNA levels in either WKY or F344 rats. Thus, the nearly absent level of CAR protein might be regulated translationally or/and post-translationally in female WKY rats.
Levels of CAR protein and mRNA in rat livers. A, preparation of total liver extracts and cytosolic fractions from untreated and PB-treated (3 h) rats, and Western blotting analysis using purified anti-human CAR antibodies were carried out as described under Materials and Methods. Lane NE contained 10 μg of total nuclear extract prepared from female mouse livers. B, liver total RNAs were prepared from untreated and PB-treated (3 h) rats. CAR mRNA was amplified using RT-PCR as described under Experimental Procedures. Various amounts (0 indicated as N, 0.5, or 50 pg) of rat CAR cDNA-containing plasmid DNA was also used as an amplification control. β-actin mRNA was amplified from the same RNA samples. Lane M contained molecular mass markers of 100-bp DNA ladders.
Discussion
Nuclear receptors have recently emerged as major mediators of xenochemical-inducible transcription of P450 genes. In addition to the well-known case of the peroxisome proliferator-activated receptor α, which trans-activates the peroxisome proliferator-response element of CYP4A genes (Johnson et al., 1996), pregnane X receptor (also called steroid and xenobiotic receptor or PAR) is identified as a trans-activator of steroids/rifampicin-response elements found in CYP3A genes (Bertilsson et al., 1998; Blumberg et al., 1998; Kliewer et al., 1998;Lehmann et al., 1998). Activating PB-inducible transcription ofCYP2B genes, CAR becomes the newest addition to a group of these nuclear receptors (Sueyoshi et al., 1999). Our present study has demonstrated that CAR is a regulatory factor for the sexually dimorphic induction by phenobarbital of CYP2B1 gene in WKY rats. A post-transcriptional modification seems to diminish the expression of CAR protein in the liver cytoplasm of female WKY rats, resulting in a severely decreased amount of nuclear CAR.
Liver-enriched CAR is a constitutively active nuclear receptor (Baes et al., 1994; Choi et al., 1997; Forman et al., 1998), and cantrans-activate PBREM without binding to PB (Kawamoto et al., 1999; Sueyoshi et al., 1999). PB elicits nuclear translocation of CAR in liver, conferring PB responsiveness to CAR in induction ofCYP2B genes. Our cDNA sequences have found no amino acid sequence differences in CARs of both sexes of WKY and F344 rats, and the CAR mRNA was equally expressed in these rats. On the other hand, the cytoplasmic CAR differed greatly at its protein level in WKY female livers, exhibiting an extremely low content compared with the other rats. This may be explained by a poor translation of CAR mRNA or by an instability of CAR protein in female WKY rats. Nuclear receptors such as glucocorticoid receptors and aryl hydrocarbon receptors exist in a large complex with such proteins as the 90-kDa heat-shock protein in cytoplasm, and dissociate from them and translocate into the nucleus upon exposure to stimuli (Wilhelmsson et al., 1990; Pongratz et al., 1992; Pratt, 1993, 1997; Smith and Toft, 1993). It is also known that these cytoplasmic receptors are constantly degraded via proteolysis in the absence of stimuli, providing an alternative mechanism by which the activity of receptors can be regulated (Dong et al., 1988; Hoeck et al., 1989; Swanson and Perdew, 1993). In this respect, the liver of WKY female rats may lack proper machinery to retain CAR in the cytoplasm so that CAR undergoes constant proteolysis. Because CAR as a protein is identical in all rats tested, a defect in the machinery must be a protein that associates with CAR or an enzyme that modifies CAR itself or the associated protein. Thus, some of the receptors other than CAR may also be affected in their stability in the cytoplasm of female WKY rats. The cytoplasmic degradation may have directly resulted in the lack of nuclear accumulation of CAR after treatment with PB, leading to poor induction of the CYP2B1 gene in female WKY rats. However, an additional possibility, whether a process of PB-elicited nuclear translocation of CAR is also impaired in the female rats, remains to be investigated. Nevertheless, further investigation using WKY rats may lead us to discover a general mechanism that causes PB and other PB-type inducers to trigger the nuclear localization of CAR.
In addition to the nuclear accumulation of CAR, CAR-mediatedtrans-activation of PBREM also differs in rats depending on the strain and sex. These differences strongly reflect an ability of NR1 to respond to PB. Because NR1 plays a major role intrans-activation of PBREM as a binding site of CAR (Honkakoski et al., 1998b), this element alone is sufficient to be activated in response to PB in HepG2 cells stably transfected with CAR-expressing plasmid (Sueyoshi et al., 1999). In male WKY rats, NR1 is still capable of being activated after PB treatment, whereas it no longer responds to PB in F344 males and females. Compared with PBREM, NR1 exhibits a high basal activity in nontreated F344 rats that seems to overwhelm the response capability of NR1 to PB. Thus, NR1 (i.e., PBREM) needs to be repressed to respond to PB. The repressor(s) may bind directly to NR1 or other elements within the PBREM, which is a composite enhancer module consisting of multiple nuclear receptor binding motifs and nuclear factor 1 (NF1) and CCAAT/enhancer-binding protein binding sites. Apparently, the repression mechanism differs between rat strains; NR1 itself may be a prime site of repression in male WKY rats, whereas a repressor may target other elements of PBREM in F344 rats. With this respect, a recent in vivo hypersensitivity study of the CYP2B2 gene has revealed that the NF1 binding site is occupied by a protein(s) in nontreated rats (Kim and Kemper, 1997). Besides NF1, we have recently found that nuclear receptors pregnane X receptor and estrogen receptor-related receptor can bind to PBREM (K. Yoshinari, I. Zelko, and M. Negishi, unpublished observations). In addition, Anderson and his associates (Stoltz et al., 1998) have shown that PB responsiveness of the PBRU can only be activated in the presence of accessory elements residing upstream or downstream of a PBRU core. Nevertheless, whether NF1 and/or other nuclear receptors are involved in repression of PBREM (therefore, attenuating PB induction) remains a focus of future investigation.
In summary, the CAR-mediated trans-activation of theCYP2B genes is conserved in mouse, rat, and human. CAR can be a regulatory factor for strain- and/or sex-dependent induction by PB of the CYP2B genes. In WKY rats, the differences in the amount of CAR in the cytoplasm seem to be the major factor for the sex-dependent induction by PB of the CYP2B1 gene, causing sex differences in metabolic capability of animal's livers. In addition, because the presence of a strong correlation between the induction of CYP2B and liver tumor promotion by PB or 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene in rats and mice has been reported (Diwan et al., 1996; Sanders and Thorgeirsson, 1999), the CAR-mediated alteration of PB responsiveness may also cause diverse pathophysiological effects. The species differences in the levels of peroxisome proliferator-activated receptor α affect differently the differences in the peroxisome proliferator-dependent carcinogenicity between rodents and humans (Holden and Tugwood, 1999). If in fact the function of CAR could also possess pathophysiological influence, species specificity and polymorphism of CAR in the human population remains a major research interest and may lead to some severe consequences in human susceptibility to toxic and carcinogenic chemicals.
Footnotes
- Received July 6, 2000.
- Accepted November 2, 2000.
-
Send reprint requests to: Dr. Masahiko Negishi, Pharmacogenetics Section, Laboratory of Reproductive and Developmental Toxicology, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709. E-mail:negishi{at}niehs.nih.gov
-
K.Y. is a Japan Society for the Promotion of Science research fellow and was supported by a grant from The Uehara Memorial Foundation (Tokyo, Japan).
-
The nucleotide sequences in this paper have been submitted to the GenBank database with accession numbers AF133094 and AF133095.
Abbreviations
- P450
- cytochrome P450
- PB
- phenobarbital
- PBREM
- phenobarbital-response enhancer module
- bp
- base pair(s)
- CAR
- constitutive active receptor
- RXR
- retinoid X receptor
- WKY
- Wistar Kyoto
- F344
- Fischer 344
- tk
- thymidine kinase
- Luc
- luciferase
- RT
- reverse transcription
- PCR
- polymerase chain reaction
- PBRU
- phenobarbital response unit
- NF1
- nuclear factor 1
- The American Society for Pharmacology and Experimental Therapeutics