Abstract
Amplification of pro-oncogenic kinases is a common genetic alteration driving tumorigenic phenotypes. Cancer cells rely on the amplified kinases to sustain cell proliferation, survival, and growth, presenting an opportunity to develop therapies targeting the amplified kinases. Utilizing small molecule catalytic inhibitors as therapies to target amplified kinases is plagued by de novo resistance driven by increased expression of the target, and amplified kinases can drive tumorigenic phenotypes independent of catalytic activity. Here, we discuss the emergence of proteolysis-targeting chimeras that provide an opportunity to target these oncogenic drivers effectively.
SIGNIFICANCE STATEMENT Protein kinases contribute to tumorigenesis through catalytic and noncatalytic mechanisms, and kinase gene amplifications are well described mechanisms of resistance to small molecule catalytic inhibitors. Repurposing catalytic inhibitors for the development of protein degraders will offer improved clinical benefits by targeting noncatalytic functions of kinases that promote tumorigenesis and overcoming resistance due to amplification.
Protein Kinase Amplification in Cancer
Kinases are essential modulators controlling cell proliferation, survival, differentiation, and migration. Mutations in kinase coding genes or chromosomal rearrangements leading to fused genes are common genetic alterations that cause cancer. Amplification of prosurvival and proproliferation kinase coding genes is another common mechanism driving cancer with a resulting increase in kinase levels and often a correlating increase in activation (Torres-Ayuso and Brognard, 2019). There are multiple examples of amplified tyrosine protein kinases playing a causal role in tumorigenesis, including the epidermal growth factor receptor (EGFR) family members, which is amplified in 30%–40% of glioblastomas and other epithelial malignancies at a lower frequency (Schlegel et al., 1994; Hynes and Lane, 2005), and ERBB2 (also known as HER2/neu), which is amplified in 15%–30% of breast cancers (Harari and Yarden, 2000; Perou et al., 2000) and approximately 10%–15% of esophageal and stomach adenocarcinomas (Cancer Genome Atlas Research Network, 2014). The fibroblast growth factor receptor 1 (FGFR1) is amplified in approximately 20% of lung squamous cell carcinomas (SCC) (Weiss et al., 2010), and the nonreceptor tyrosine kinase, focal adhesion kinase (FAK, PTK2) is amplified in 25% of ovarian serous cystadenocarcinoma (Ward et al., 2013). Amplification is also frequent among serine/threonine protein kinases. For instance, cyclin dependent kinase (CDK) 4 is amplified in 15% of sarcomas and glioblastomas (Reifenberger et al., 1994), CDK6 in 12% of esophageal adenocarcinomas (Cancer Genome Atlas Research Network et al., 2017), and CRAF (RAF1) in 10% of bladder carcinomas (Bekele et al., 2021).
Multiple kinases are often coamplified, as their coding genes are members of large chromosomal regions that are frequently amplified in cancer. For example, distal amplification of the long arm of the third chromosome (3q26-29) is highly prevalent in SCC arising in different tissues, including lung, head and neck, and esophageal SCC. The 3q amplicon contains multiple kinase coding genes, such as PIK3CA (phosphatidylinositol 3-kinase catalytic subunit p110alpha), PRKCI [Protein Kinase C (PKC) iota], Mitogen Activated Kinase Kinase Kinase 13 (MAP3K13) [Leucine Zipper-Bearing Kinase (LZK)], and TNIK (TRAF2- and NCK-interacting kinase) (Bensen and Brognard, 2021). In some instances, a kinase regulatory protein is amplified instead of the kinase coding gene. For example, the regulatory cyclin D1 gene (CCND1), is a positive regulator of CDK4 and CDK6, and is amplified in one-third and one-quarter of esophageal adenocarcinomas and head and neck SCC, respectively. These different scenarios lead to upregulation of the associated kinase expression or activity.
Cancer cells rely on the upregulated kinase levels or activity to sustain cell survival and uncontrolled cell proliferation. Therefore, amplified kinases constitute genetic vulnerabilities for precision medicine based therapeutic intervention strategies. As a result, several small molecule catalytic inhibitors are approved or under development to target amplified kinase drivers in cancer (Gross et al., 2015). The following considerations need to be acknowledged for efficient targeting of amplified kinase drivers. First, kinases can contribute to tumorigenesis through activity-dependent and -independent mechanisms. Catalytic-independent or scaffolding functions of amplified kinases might have a relevant contribution to tumorigenesis since gene amplification might lead to an increase in protein levels but not necessarily to increased kinase activity. For example, the tyrosine kinase FAK acts as a scaffold in the nucleus to modulate the activity of certain transcriptional complexes (Dawson et al., 2021). A FAK degrader has been used to demonstrate that FAK promotes migration and invasion of a breast cancer cell line model through catalytic-independent mechanisms (Cromm et al., 2018). We have recently characterized amplified TNIK as a targetable vulnerability in lung SCC and demonstrated that TNIK inhibitors efficiently reduce tumor growth. Nonetheless, we identified lung SCC cell lines sensitive to TNIK depletion mediated by shRNA but not to treatment with a small molecule inhibitor, suggesting that TNIK might contribute to tumorigenesis through an activity-independent mechanism (Torres-Ayuso et al., 2021). EGFR, CDK6, and several additional kinases also display catalytic-independent functions (Rauch et al., 2011). Therefore, unless noncatalytic tumor-promoting functions are suppressed by inhibiting the kinase’s catalytic activity or rely on a specific kinase conformation that could be affected by inhibitor binding, catalytic-independent mechanisms will not be affected by treatment with small molecule catalytic inhibitors. These kinase activity-independent mechanisms can still sustain cell proliferation and survival, making cells refractory or resistant to catalytic-inhibitor treatment. Our understanding of the catalytic-independent oncogenic mechanisms of kinases is still limited.
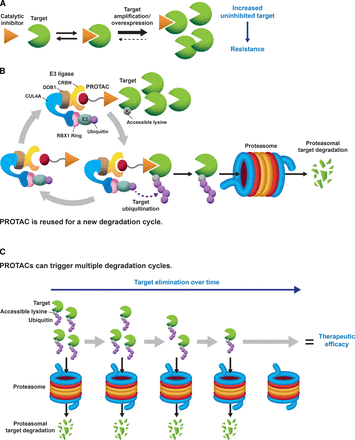
Besides kinase activity-independent mechanisms of tumorigenesis, cancers driven by amplified kinases might quickly adapt and develop resistance against small molecule catalytic inhibitors. Indeed, gene amplification and subsequent target overexpression is a described mechanism of resistance to small molecule catalytic inhibitors targeting oncogenic BRAF, EGFR, BCR-ABL1, and echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) (Corcoran et al., 2010; Lovly and Shaw, 2014). In this case, overexpression of the target might displace the inhibitory equilibrium toward reduced target occupancy; thus, higher concentrations of the inhibitor would be necessary to achieve therapeutic efficacy but likely yield potential toxicity issues (Fig. 1A). Therefore, new approaches are required to target amplified kinase drivers effectively.
(A) Catalytic inhibitors must occupy most of their intended kinase to achieve therapeutic efficacy (i.e., “occupancy-driven” pharmacology). Kinase gene amplification or overexpression can balance the equilibrium toward the accumulation of an uninhibited kinase pool, and cancer cells become resistant to treatment with catalytic inhibitors. (B) PROTACs are heterobifunctional molecules that bring into close proximity a protein (kinase) of interest with an E3 ubiquitin ligase. When the intended protein kinase and the ubiquitin ligase complex interact in the right orientation, and an “acceptor” lysine is available on the target surface, the protein kinase becomes polyubiquitinated and is targeted for proteasomal degradation (“event-driven” pharmacology). The PROTAC gets recycled to start a new degradation reaction. The CRL4-Cereblon ubiquitin ligase complex has been used as an example. (C) PROTACs can trigger multiple cycles of degradation and be efficacious therapies at low target-to-compound or substoichiometric ratios. For amplified kinases, the kinase will be eliminated over time, and therapeutic benefit will be achieved.
Targeted Protein Degradation
Targeted protein degradation is an emerging field for therapeutic intervention that leverages the cellular machinery responsible for protein quality control (i.e., appropriate folding) to target cancer drivers for degradation. Proteolysis targeting chimeras (PROTACs) constitute one of these novel modalities and take advantage of the protein ubiquitination and proteasomal degradation system. PROTACs are heterobifunctional molecules consisting of a pharmacophore that recognizes a protein of interest or target, an E3 ubiquitin ligase warhead, and a linker. Upon PROTAC treatment, the target is brought in proximity to an E3 ligase forming a ternary complex: target-PROTAC-ubiquitin ligase. For the PROTAC to work efficiently, the target needs to be engaged in an optimal orientation with an “acceptor lysine” on the surface of the protein target being exposed for ubiquitination. The ubiquitinated protein of interest is subsequently degraded via the proteasome and the PROTAC is recycled to catalyze a new degradation cycle for the target (Fig. 1B) (Nalawansha and Crews, 2020). Most PROTACs are based on engaging the von Hippel-Lindau Cullin 2-Ring Ubiquitin Ligase (CRL2-VHL), CRL4-Cereblon (CRL4-CRBN), inhibitors of apoptosis (IAPs), and Murine Double Minute (MDM) 2 E3 ubiquitin ligases; however, there are ongoing efforts to expand the number of recruited E3 ligases for PROTAC generation that include the identification of novel ligands (Nalawansha and Crews, 2020; Luo et al., 2021). Based on the same principles as PROTACs, lysosome targeting chimeras have been generated to target extracellular proteins to the lysosome where the protein of interest is degraded (Banik et al., 2020).
Among the different classes of proteins, kinases are primed for the generation of protein degraders because of the existing number of catalytic inhibitors that can be repurposed for PROTAC development (Table 1). There are several examples of small molecule kinase inhibitors that have been used as pharmacophores for the generation of novel PROTACs. For example, PROTACs that effectively degrade ABL1 and the oncogenic fusion protein BCR-ABL1, the main driver in chronic myeloid leukemia, have been designed from the ATP-competitive ABL1 inhibitors Bosutinib and Dasatinib, as well as the allosteric ABL1 inhibitor GNF-5 (Lai et al., 2016; Burslem et al., 2019). Similarly, the ALK inhibitors Ceritinib and TAE684 were the base for the generation of ALK and ALK-harboring fusion protein degraders (Powell et al., 2018). Additional small molecule inhibitors have been successfully converted into PROTACs (revised in Sun et al., 2019). Examples include the generation of PROTACs against EGFR and HER2 derived from Lapatinib, Gefitinib, and Afatinib (Burslem et al., 2018a); a FAK PROTAC that includes the FAK inhibitor from Defactinib (Cromm et al., 2018); and a BRAF-V600E-specific PROTAC using Vemurafenib as a pharmacophore (Alabi et al., 2021), etc.
List of PROTACs targeting oncogenic lipid and protein kinases
Revised in (Sun et al., 2019) and (Yu et al., 2021) and searched from Pubmed (pubmed.ncbi.nlm.nih.gov) as of December 2021.
Because of their mechanism of action of degrading a protein target, PROTACs could be more advantageous than small molecule inhibitors at targeting cancer drivers, including amplified kinases. As discussed above, kinases can contribute to tumorigenesis through catalytic and noncatalytic mechanisms; the latter might be unaffected by catalytic inhibitors. Catalytic inhibitors must occupy almost all of the existing target molecules to be effective therapies [i.e., “occupancy-driven” pharmacology (Lai and Crews, 2017)], as a small uninhibited kinase pool could still sustain its pro-oncogenic mechanisms (Fig. 1A). Against the “occupancy-driven” mechanism of small molecule catalytic inhibitors, PROTACs reduce the number of molecules for their intended target, and get recycled to trigger additional degradation events [i.e., “event-driven” pharmacology (Lai and Crews, 2017)]. Thus, PROTACs reduce their target expression and downregulate both catalytic and noncatalytic functions of kinases (Fig. 1B).
Supporting the notion above, several PROTACs have shown enhanced activity when compared with their parental small molecule catalytic inhibitors. For example, a Defactinib-derived FAK PROTAC showed higher activity than Defactinib in reducing FAK downstream signaling as well as in inhibiting the migration and invasion of the MDA-MB-231 breast cancer cell line, indicating that FAK contributes to the migration and invasion of these cells through both catalytic-dependent and -independent functions (Cromm et al., 2018). Similarly, an Ibrutinib-derived Bruton’s Tyrosine Kinase (BTK) targeting PROTAC showed enhanced efficacy to reduce cell viability compared with the parental compound (Sun et al., 2018), and Receptor Tyrosine Kinase-directed PROTACs were also more efficient than similar compounds that only inhibit catalytic activity (Burslem et al., 2018a). These findings are not restricted to tyrosine kinases; for example, a BRAF VHL-based PROTAC, SJF-0628, outperformed the Vemurafenib and a negative control compound that does not trigger BRAF degradation (SJF-0661, that has an inverted stereocenter in the critical hydroxyl-proline group in the VHL ligand), in reducing the viability of cells expressing mutant-BRAF (Alabi et al., 2021). Similar observations were made with a Cereblon-based, mutant-BRAF selective PROTAC (Posternak et al., 2020), where the PROTAC decreased cell viability to a higher extent than the methylated-Cereblon ligand control compound that does not degrade mutant-BRAF, but still inhibits the catalytic activity of BRAF. Lastly, a CDK9 PROTAC also had increased cytotoxic activity compared with CDK9 inhibition alone (Olson et al., 2018), suggesting that this might be a general phenomenon of kinase-degrading PROTACs.
Moreover, a single molecule of PROTAC can catalyze several degradation cycles (Paiva and Crews, 2019), and thus, PROTACs can trigger their intended target degradation at substoichiometric doses (Bondeson et al., 2015; Lu et al., 2015; Olson et al., 2018). Therefore, PROTACs might be effective pharmacological agents at a low compound-to-target ratio (Fig. 1, B and C). This property of PROTACs is especially relevant in the context of kinase gene amplification or overexpression, which are acknowledged mechanisms of resistance to small molecule catalytic inhibitors since these alterations balance the equilibrium toward the accumulation of the untargeted kinase. It has been shown that converting a kinase inhibitor into a PROTAC provides a higher level of selectivity in comparison with the parental compound, since only a limited number of the PROTAC-interacting kinases gets degraded (Bondeson et al., 2018; Tovell et al., 2019b; Donovan et al., 2020). For example, using the promiscuous inhibitor Foretinib, Bondeson and colleagues (2018) found that of 54 protein kinases that were Foretinib targets, only 9 kinases were degraded by a Foretinib-based VHL-engaging PROTAC, whereas 14 kinases were degraded by a Cereblon-engaging PROTAC (Bondeson et al., 2018). Together, the capacity of PROTACs to trigger several cycles of degradation and show enhanced specificity could make kinase targeting PROTACs more potent and selective weapons for cancer treatment than their parental catalytic inhibitors (Fig. 1C, Table 2).
Comparison of small molecule ATP-competitive catalytic inhibitors versus PROTACs
Optimizing PROTAC-Mediated Kinase Degradation: A Windy Road
Despite myriad existing PROTACs against kinases, PROTAC design and generation remains an empirical process. Different groups have studied the effect of several variables on efficient target degradation and PROTAC selectivity to inform drug design strategies, including the dependence on PROTAC engagement and binding affinity to the target, the composition and structure of the linker, the formation of a stable ternary complex, the recruitment of different E3 ligases, and the abundance of the target (Pettersson and Crews, 2019; Riching et al., 2018; Donovan et al., 2020). Multiple lines of evidence indicate that target engagement is insufficient for effective degradation. For example, several PROTACs were designed against ABL1 from the small molecule inhibitors Imatinib, Dasatinib, and Bosutinib. All the PROTACs were able to engage ABL1, whereas none of the Imatinib-based PROTACs triggered degradation of ABL1 or BCR-ABL1 (Lai et al., 2016). Similarly, PROTACs have been generated based on the CDK4/6 inhibitor Palbociclib; however, the Palbociclib-derived PROTACs only triggered CDK6 degradation and did not affect CDK4 levels (Brand et al., 2019; Rana et al., 2019; Su et al., 2019).
Studies with multikinase inhibitors have shown that the binding affinity of the PROTAC to its target does not predict efficient target degradation; instead, the stability of the ternary complex target-PROTAC-E3 ligase was a better predictor of target degradation (Bondeson et al., 2018). Indeed, the selectivity of CDK6 degraders versus CDK4, or PROTACs triggering degradation of mutant-BRAF but not of the wild-type proteins relies on the lack of formation of a stable ternary complex for targets that are not degraded (Brand et al., 2019; Alabi et al., 2021). A recent study analyzing the degradability potential of kinases or “degradable kinome” indicates that the formation of a stable ternary complex does not fully predict the efficacy of the degradation catalysis, as kinase degradation could be observed without detecting the ternary complex, suggesting a rapid degradation kinetic (Donovan et al., 2020). Indeed, the formation of a ternary complex is insufficient to engage the target’s degradation. For example, p38-MAPK-alpha- and delta-isoform-specific PROTACs, SJF-alpha and SFJ-delta, respectively, were developed from the inhibitor Foratenib by using different linker lengths and attaching the resulting compound to two different positions in the VHL ligand (Smith et al., 2019). Interestingly, both PROTACs could trigger the formation of a ternary complex with p38 MAPK delta, whereas efficient p38-MAPK-delta degradation was only observed with the SFJ-delta PROTAC (Smith et al., 2019). Therefore, the structural characteristics of the interaction interface between the target kinase and the recruited E3 ligase also impact target degradation and can be exploited to provide additional PROTAC selectivity.
The engagement of different E3 ligases also determines PROTAC efficacy and selectivity, even when sharing the same pharmacophore. For instance, a Dasatinib-based PROTAC coupled to a VHL ligand degraded ABL1 but failed to downmodulate BCR-ABL1 levels, whereas when Dasatinib is coupled to the Cereblon ligand, this PROTAC triggered the degradation of both ABL1 and BCR-ABL1 (Lai et al., 2016). These observations have been further confirmed in recent high throughput studies using promiscuous kinase inhibitors as pharmacophores (Bondeson et al., 2018; Huang et al., 2018; Donovan et al., 2020). For example, the use of a VHL- or Cereblon-coupled PROTAC from the multikinase inhibitor Foretinib showed that although both PROTACs efficiently degrade several common targets, each E3-ligase-coupled PROTAC selectively triggers the degradation of a specific subset of kinases (Bondeson et al., 2018).
Lastly, the linker length and composition can significantly affect the efficiency of degradation of a given target (Crew et al., 2018; Smith et al., 2019). Within kinases, it has been shown that although some members are permissive toward different linkers for efficient target degradation, a subset of kinases have a strong preference toward short or long linker lengths or even to different linker attachment regioselectivity (Donovan et al., 2020). In addition, the linker length can also impact the PROTAC specificity. One of the earliest examples of such contribution of the linker length to a degrader specificity was observed with Lapatinib-derived PROTACs; it was reported that by modifying the linker length from two polyethylene glycol molecules to three, the PROTAC could be converted from a dual EGFR-and-HER2 degrader to a specific EGFR PROTAC (Burslem et al., 2018a).
PROTACs as Tools to Inform New Cancer Biology
As mentioned earlier, multiple protein kinases display catalytic-independent mechanisms of action (Rauch et al., 2011), and these functions might not be affected by treatment with small molecule catalytic inhibitors. Affecting both activity-dependent and -independent functions of protein kinases might be the underlying reason why certain PROTACs show increased activity compared with their parental compounds or molecules with similar properties that do not trigger their intended target degradation (Cromm et al., 2018; Olson et al., 2018; Burslem et al., 2018a; Posternak et al., 2020; Alabi et al., 2021). Therefore, PROTACs, in comparison with catalytic inhibitors or their control compounds that retain their inhibitory capacity but do not degrade the protein target, in combination with technologies such as mass spectrometry (i.e., phosphoproteomics) or RNA-seq, can be used as tools differentiate catalytic versus noncatalytic mechanisms of tumorigenesis promoted by oncogenic protein kinases and shed light on new tumor biology. For example, pathways controlled in a kinase activity-dependent manner will be affected by treatment with either a catalytic-inhibitor or a PROTAC, whereas downstream effectors that rely on scaffolding functions, will mainly be only downregulated after PROTAC-mediated kinase degradation. The use of PROTACs can have advantages over genetic systems with similar outcomes, such as RNAi, since PROTACs diminish protein levels in a rapid and dose-controlled manner, avoiding issues such as emergence of compensatory pathways (Burslem and Crews, 2020). Importantly, for targets for which generating a PROTAC might be challenging, alternative approaches such as using the CRISPR-Cas9 gene editing technology to tag proteins with either the HaloTag, the FKBP12F36V, a bromodomain, or Green Fluorescent Protein (GFP), can allow selective protein degradation using HaloPROTACs (Buckley et al., 2015; Tovell et al., 2019a), the dTAG (Nabet et al., 2018, 2020), BromoTAG (Bond et al., 2021), or the affinity-directed protein missile (AdPROM) (Fulcher et al., 2016; Simpson et al., 2020) systems.
PROTACs have already contributed to reveal new functions of certain protein kinases. For example, using PROTACs, a new role for the Aurora kinase A (AURKA) in the cell cycle has been discovered (Adhikari et al., 2020). Although Aurora kinase A inhibition causes a G2/M arrest, its degradation arrested cells in the S phase. This new observation is likely the result of the interaction between Aurora kinase A and proteins that participate in RNA metabolism, that are not Aurora kinase A substrates. Further investigation is required to address the exact mechanism (Adhikari et al., 2020). Similarly, the use of PROTACs has uncovered noncatalytic functions for several protein kinases, including BCR-ABL1 in Chronic Myeloid Leukemia (Burslem et al., 2019), FAK in the control of migration and invasion (Cromm et al., 2018), or CDK6 in Philadelphia-positive acute lymphoblastic leukemia (De Dominici et al., 2020). Follow up studies will further reveal the importance of these noncatalytic mechanisms in promoting different cancer-associated phenotypes.
PROTACs as Anticancer Agents: The Road Ahead
Several lines of evidence highlight that PROTAC-mediated protein degradation could be a more efficacious strategy for cancer treatment than small molecule catalytic inhibitors, as discussed above, especially for difficult-to-target drivers, which include amplified oncogenes (including kinases) and transcription factors. Although there is increasing evidence of PROTACs being efficient in vivo, including from clinical trials (for example, the androgen receptor and estrogen receptor degrading PROTACs ARV-110 and ARV-471, respectively), little is known about PROTAC biodistribution and metabolism, and PROTACs may present some disadvantages when compared with small molecule catalytic inhibitors (Table 2). The main efforts in transitioning PROTACs for in vivo applications have focused on improving their pharmacokinetic and pharmacodynamic properties. PROTACs do not follow Lipinski’s rule of 5 (Lipinski et al., 2001); for example, PROTACs are usually molecules with molecular weights higher than 500 Da and with more than five hydrogen bond donors. Indeed, this high molecular weight negatively impacts the PROTACs solubility and permeability, and modifications on the PROTAC structure might be required to solve these issues and improve PROTACs biodistribution (Cecchini et al., 2021). Some of these issues could be overcome by developing new classes of targeted degraders, such as the “molecular glues”, which are compounds with molecular weights similar to that of catalytic inhibitors that join the interfaces of a protein of interest with an ubiquitin ligase to promote the protein degradation via the proteasome system (Kozicka and Thoma, 2021). Indeed, molecular glues targeting protein kinases, specifically CDK12-cyclin K, have been reported (Lv et al., 2020; Słabicki et al., 2020; Dieter et al., 2021).
PROTAC metabolism has recently been evaluated in a study conducted by Goracci and colleagues (2020). The authors concluded that the linker was the main contributor to the PROTAC metabolic stability and showed that CYP3A4 (cytochrome P450 family 3 subfamily A member 4) can play an essential role in PROTAC degradation, and that human aldehyde oxidase could also metabolize PROTACs with the VHL ligand.
One potential advantage of PROTACs as therapeutics relies on their “event-driven” mechanism of action. This property has been recently explored with a Receptor Interacting Serine/Threonine Kinase 2 (RIPK2) PROTAC (Mares et al., 2020). In this study, the authors demonstrated a disconnection between the pharmacokinetic and pharmacodynamic properties of the selected PROTAC, with the PROTAC efficiently degrading RIPK2 even when it was not detectable and supporting the notion that PROTAC could be efficacious at low doses and reduced tissue exposure. However, it is acknowledged in this study that RIPK2 displays a slow resynthesis rate, indicating that additional studies will be necessary.
Besides the pharmacological properties of PROTACs and based on clinical experience with small molecule catalytic inhibitors, it is necessary to address whether cancer cells could become resistant to PROTACs. For example, cancer cells can develop mutations that reduce the pharmacophore’s binding affinity to its intended target (Lovly and Shaw, 2014; Torres-Ayuso and Brognard, 2019); consistent with this mechanism of resistance, mutations in the protein of interest can render it resistant to PROTAC-mediated degradation, as recently shown for CDK12 (Jiang et al., 2021). Nonetheless, because target degradation efficacy does not correlate with the PROTAC binding affinity to its target, PROTACs could still effectively degrade these novel variants. This has been demonstrated with an Ibrutinib-based BTK PROTACs, MT-802 (Buhimschi et al., 2018), and P13I (Sun et al., 2018), which effectively degrade both the wild-type BTK and the Ibrutinib-resistant C481S BTK mutant. Of note, these PROTACs lack the Ibrutinib’s acrylamide moiety that covalently binds BTK C481, enabling these degraders to target both wild-type- and C481S-mutant BTK and retain a catalytic mechanism of action (Buhimschi et al., 2018). In fact, irreversible PROTAC-covalent binding to BTK can impair BTK degradation, since these PROTACs do not get recycled (Tinworth et al., 2019), although covalent-reversible BTK PROTACs can efficiently degrade their intended target with some advantages such as increased selectivity and intracellular retention time (Gabizon et al., 2020; Guo et al., 2020).
Resistance to PROTAC treatment could also emerge by changes in the protein degradation system; these alterations usually involve the loss of core components of the degradation machinery rather than mutations in their respective coding genes (Zhang et al., 2019; Shirasaki et al., 2021). Notably, the alterations that trigger resistance to PROTACs are different depending on the engaged E3 ligase and suggest that sequential treatment with PROTACs engaging other E3 ligases could be an approach to prevent or delay resistance to protein degradation (Ottis et al., 2019; Farnaby et al., 2021; Shirasaki et al., 2021). With PROTACs entering clinical trials for evaluation in solid and hematologic malignancies (Mullard, 2021) (Table 3), their true potential as anticancer agents will be unveiled.
List of PROTACs or heterobifunctional degraders in clinical trials (accessed from clinicaltrials.gov as of December 2021)
Conclusions and Future Perspectives
Targeted protein degradation is an emerging and evolving therapeutic option, especially for drivers that are difficult to target through conventional approaches, including amplified kinases. Kinase catalytic inhibitors can be easily incorporated into protein degraders or PROTACs; however, there is some discrepancy between the affinity of a small molecule and the degradation efficacy of its derived PROTAC, which is not fully understood. Some studies claim that such discrepancy might result from the stability of the ternary target-PROTAC-E3 ligase complex, the retention time of the PROTAC, or the geometry of the ternary complex not allowing the target ubiquitination. Despite these challenges, when a PROTAC can trigger the degradation of their intended target, it is expected that they will be superior to catalytic inhibitors in suppressing the function of amplified oncogenic kinase drivers as they work in substoichiometric doses and will abrogate both catalytic and noncatalytic functions of the oncogenic kinase (Fig. 1C). The possibility of generating PROTACs engaging different E3 ligases to degrade the same oncogenic target will likely reduce the probability that cancer cells become resistant to degradation of a protein of interest, although in vivo and clinical evidence is still required. Ongoing and future clinical trials using PROTACs and other protein degrading approaches will shed light on the potential of these novel therapeutics to become the therapy of choice in precision oncology approaches.
Acknowledgments
The authors thank Allan Kane from the Frederick National Laboratory for Cancer Research for assistance in illustration preparation.
Authorship Contributions
Participated in research design: Torres-Ayuso, Brognard.
Wrote or contributed to the writing of the manuscript: Torres-Ayuso, Brognard.
Footnotes
- Received April 19, 2021.
- Accepted January 27, 2022.
Funding for this work is supported by the National Cancer Institute [Grant ZIABC011691], and the Intramural Research Program of the National Institutes of Health through an NCI FLEX award (to J.B.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The authors declare no potential conflicts of interest.
Abbreviations
- ALK
- anaplastic lymphoma kinase
- BTK
- Bruton’s Tyrosine Kinase
- CDK
- cyclin dependent kinase
- EGFR
- epidermal growth factor receptor
- EML4
- echinoderm microtubule-associated protein-like 4
- FAK
- focal adhesion kinase
- PROTAC
- proteolysis targeting chimera
- SCC
- squamous cell carcinomas
- TNIK
- TRAF2- and NCK-interacting kinase
- U.S. Government work not protected by U.S. copyright.