Abstract
Agonist-induced endocytosis is a key regulatory mechanism for controlling the responsiveness of the cell by changing the density of cell surface receptors. In addition to the role of endocytosis in signal termination, endocytosed G protein–coupled receptors (GPCRs) have been found to signal from intracellular compartments of the cell. Arrestins are generally believed to be the master regulators of GPCR endocytosis by binding to both phosphorylated receptors and adaptor protein 2 (AP-2) or clathrin, thus recruiting receptors to clathrin-coated pits to facilitate the internalization process. However, many other functions have been described for arrestins that do not relate to their role in terminating signaling. Additionally, there are now more than 30 examples of GPCRs that internalize independently of arrestins. Here we review the methods, pharmacological tools, and cellular backgrounds used to determine the role of arrestins in receptor internalization, highlighting their advantages and caveats. We also summarize key examples of arrestin-independent GPCR endocytosis in the literature and their suggested alternative endocytosis pathway (e.g., the caveolae-dependent and fast endophilin-mediated endocytosis pathways). Finally, we consider the possible function of arrestins recruited to GPCRs that are endocytosed independently of arrestins, including the catalytic arrestin activation paradigm. Technological improvements in recent years have advanced the field further, and, combined with the important implications of endocytosis on drug responses, this makes endocytosis an obvious parameter to include in molecular pharmacological characterization of ligand-GPCR interactions.
SIGNIFICANCE STATEMENT G protein–coupled receptor (GPCR) endocytosis is an important means to terminate receptor signaling, and arrestins play a central role in the widely accepted classical paradigm of GPCR endocytosis. In contrast to the canonical arrestin-mediated internalization, an increasing number of GPCRs are found to be endocytosed via alternate pathways, and the process appears more diverse than the previously defined “one pathway fits all.”
Introduction
G protein–coupled receptors (GPCRs) form the largest class of transmembrane cell surface receptors, and they regulate intracellular signaling in response to a diverse range of extracellular stimuli (Lefkowitz, 2013). Initiation, processing, and termination of these signals are tightly regulated in a spatiotemporal manner to ensure homeostasis. To date, approximately one-third of the marketed drugs target GPCRs, illustrating their importance in human pathologies (Hauser et al., 2017).
Agonist-stimulated GPCRs catalyze the activation of heterotrimeric G proteins and thereby modulate downstream effector proteins, including adenylyl cyclase, phospholipase C, and Rho guanine nucleotide exchange factor (Hilger et al., 2018). Stimulation of these pathways can evoke receptor phosphorylation on serine and threonine residues in the third intracellular loop (ICL) and the carboxy-terminal tail (C-tail), mediated by GPCR kinases (GRKs) and second messenger–dependent kinases from the protein kinase A and protein kinase C (PKC) families (Ferguson, 2001; Gurevich and Gurevich, 2019). Recruitment of arrestin proteins to phosphorylated active-state receptors can lead to desensitization of second messenger signaling by sterically precluding G protein coupling to receptors. Furthermore, arrestins can facilitate and regulate receptor signaling by scaffolding a wide range of proteins involved in signaling pathways, for instance, components of mitogen-activated protein kinase cascades, Src family tyrosine kinases, and E3 ubiquitin ligases (Peterson and Luttrell, 2017).
To protect cells from overstimulation upon prolonged or repeated exposure to agonists, activated GPCRs are removed from the cell surface by means of endocytosis. In this way, the majority of the cell surface population of a GPCR may be internalized within minutes of agonist stimulation (January et al., 1997). Internalized receptors are processed and sorted in the endosomal network for recycling to the plasma membrane (resensitization) or degradation via the lysosomal pathway (downregulation) (Pavlos and Friedman, 2017). Moreover, GPCRs can signal from intracellular compartments as well. This may result in different signaling consequences compared with cell surface signaling due to distinct location and timing (Lobingier and von Zastrow, 2019).
In this mini review we summarize the endocytosis pathways that have been described for GPCRs—arrestin-dependent and -independent—and the receptors that have been shown to be endocytosed independently of arrestins, including the tools and methods that have been used to assess the arrestin dependence. Finally, we discuss the possible functional implications of arrestin recruitment to receptors that internalize independently of arrestins.
Clathrin-Mediated Endocytosis
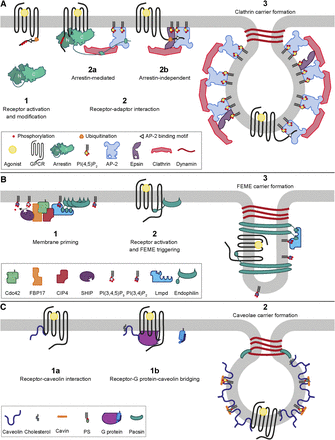
Several mechanisms of GPCR endocytosis have been described. The best characterized endocytic route and the predominant pathway of endocytosis in mammalian cells is clathrin-mediated endocytosis (Pearse, 1976) (Fig. 1A). Ligand-activated GPCRs are in many cases targeted to clathrin-coated pits by binding to arrestins (Traub, 2009). There are four subtypes of arrestin: arrestin-1 and -4 that are mainly expressed in the visual system and arrestin-2 and -3 (also called β-arrestin-1 and -2, respectively) that are ubiquitously expressed (Mundell et al., 2002). Activated arrestins are recruited to clathrin-coated pits through interaction with the β2 adaptin subunit of adaptor protein-2 (AP-2), via an RXR motif located in the autoinhibitory C-tail of all arrestin subtypes (Laporte et al., 2000; Schmid et al., 2006). In addition, arrestins can interact with clathrin through an LIELD or LIEFE motif located in the autoinhibitory segment of arrestin-2 and -3 and/or through a loop in the C-terminal domain (C-domain) of arrestin that is present in the long splice isoform of arrestin-2 and in arrestin-1 and -4 (Goodman et al., 1996; Kang et al., 2009). In the inactive form, the C-tail of arrestin functions as an autoinhibitory segment that is bound to a groove in the N-terminal domain (N-domain) of arrestin, thus masking the AP-2 and clathrin motifs (Hirsch et al., 1999). Binding of a phosphorylated receptor C-tail displaces the autoinhibitory segment and enables arrestin to interact with AP-2 and clathrin (Xiao et al., 2004; Nobles et al., 2007). Alternatively, the µ2 adaptin subunit of AP-2 has been shown to interact directly with GPCRs, through which it might facilitate clathrin-mediated endocytosis independently of arrestins (Diviani et al., 2003; Paing et al., 2006).
Endocytosis pathways involved in GPCR internalization. (A) Clathrin-mediated endocytosis of GPCRs. (1) Ligand-mediated receptor activation leads to conformational changes, for instance, exposing AP-2 binding motifs in the receptor C-tail, and/or post-translational modification of the receptor, such as phosphorylation or ubiquitination. (2a) Most GPCRs are recruited to clathrin-coated pits through arrestins. Binding of arrestin to the phosphorylated receptor C-tail enables interaction of the arrestin C-tail with AP-2 and clathrin. (2b) Additionally, receptors can be recruited to clathrin-coated pits independently of arrestins, through direct interaction with AP-2 or binding of their polyubiquitinated C-tails to epsins. (3) Clathrin is recruited to the plasma membrane by adaptor proteins, such as AP-2 and epsins, to form clathrin carriers upon scission of clathrin-coated pits from the plasma membrane by dynamin. (B) Fast endophilin-mediated endocytosis. (1) Endophilin is concentrated at the plasma membrane through interaction with lamellipodin (Lmpd), which depends on the sequential action of Cdc42, formin-binding protein 17 (FBP17), CIP4, and phosphatidylinositol 3,4,5-trisphosphate 5′ phosphatase (SHIP) 1/2. (2) Interaction of a ligand-activated GPCR with endophilin triggers FEME. (3) FEME carriers are formed upon scission of endophilin stabilized invaginations by dynamin. (C) Endocytosis via caveolae. GPCRs can be recruited to caveolae through direct interaction with caveolin (1a) or via Gαq-mediated interaction with caveolin (1b). (2) Caveolae membrane deformations consist of oligomeric caveolin complexes stabilized by coat-forming cavins and membrane curvature inducing pacsins. Caveolae carrier formation relies on dynamin for membrane scission. For clarity, only a selection of the components involved in the various endocytosis pathways are represented in the schematic. PI(3,4)P2, phosphatidylinositol 3,4-bisphosphate; PI(3,4,5)P3, phosphatidylinositol 3,4,5-trisphosphate; PS, phosphatidylserine.
AP-2 and other clathrin adaptor proteins initiate clathrin-mediated endocytosis by binding to plasma membrane domains enriched for the phospholipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. AP-2 is a tetrameric protein complex comprising α, β2, μ2, and σ2 adaptin subunits. Interaction with PI(4,5)P2 induces a conformational change in AP-2, exposing binding sites for clathrin, receptors, and other cargo proteins and additional PI(4,5)P2 interaction motifs (Kelly et al., 2014) (Fig. 1A, step 2a and b). Clathrin molecules interact with AP-2 and form polymeric lattices, enveloping the plasma membrane into clathrin-coated pits (Fotin et al., 2004; Dannhauser and Ungewickell, 2012) (Fig. 1A, step 3). Bin/amphiphysin/Rvs (BAR) domain–containing proteins, such as endophilins, induce and stabilize membrane curvature and thereby mediate constriction of the invagination neck, whereas membrane scission is powered by oligomers of dynamin, which is a GTPase (Sundborger et al., 2011). Dephosphorylation of PI(4,5)P2 (Cremona et al., 1999) and chaperone-mediated disruption of clathrin-clathrin interactions (Schlossman et al., 1984) drive the uncoating of clathrin-coated vesicles, followed by vesicle fusion to early endosomes.
Another class of clathrin adaptors is constituted by epsins, which are monomeric proteins that can interact simultaneously with PI(4,5)P2, clathrin, and AP-2 (Traub, 2009). Through their ubiquitin-interacting motifs, epsins can directly recruit polyubiquitinated proteins to clathrin-coated pits, including GPCRs (Chen et al., 2011) (Fig. 1A, step 2b). Many other proteins contribute to the formation of clathrin-coated pits (Traub, 2011), potentially including yet unidentified GPCR adaptor proteins.
Clathrin-Independent Endocytosis
Although the majority of endocytic vesicles arise from clathrin-mediated endocytosis (Pearse, 1976; Anderson et al., 1977), several other endocytic mechanisms have been shown to operate at the plasma membrane of mammalian cells. Some of these have been implicated in the internalization of GPCRs. Characterization of clathrin-independent endocytosis pathways, however, is hampered by a lack of knowledge about specific cargo and cellular machinery and an absence of pathway-specific manipulation tools (Sandvig et al., 2018). Hence, clathrin-independent endocytosis routes are generally less well delineated than clathrin-mediated endocytosis.
Fast Endophilin-Mediated Endocytosis.
In addition to their contribution to clathrin-mediated endocytosis, endophilins (endophilin A1–3, but not B1 and B2) are essential for a rapid, clathrin-independent internalization pathway, called fast endophilin-mediated endocytosis (FEME) (Boucrot et al., 2015) (Fig. 1B). This rapid response to receptor activation (few seconds) relies on the presence of endophilin-enriched domains in the plasma membrane, which are dynamically assembled and disassembled in the absence of activated receptors or other cargo proteins.
FEME priming starts with the recruitment of the FES/CIP4 homology–BAR domain proteins formin-binding protein 17 and Cdc42-interacting protein 4 (CIP4) by active, membrane-bound cell division control protein 42 homolog (Cdc42). In turn, these proteins recruit the phosphatidylinositol 3,4,5-trisphosphate 5′ phosphatase 1 and 2, thus increasing the local concentration of phosphatidylinositol-3,4-bisphosphate (Chan Wah Hak et al., 2018). Through its phosphatidylinositol-3,4-bisphosphate–binding pleckstrin homology domain and multiple endophilin-binding motifs, lamellipodin facilitates the concentration of endophilins in patches at the cell surface (Vehlow et al., 2013; Boucrot et al., 2015) (Fig. 1B, step 1). Endophilins containing BAR domains with an additional N-terminal amphipathic helix induce plasma membrane curvature by insertion of amphipathic helices in the inner membrane leaflet as well as membrane scaffolding through their BAR domains (Boucrot et al., 2012), whereas protein scaffolding is mediated via their Src homology 3 (SH3) domains (Boucrot et al., 2012, 2015; Vehlow et al., 2013). FEME vesicle formation requires membrane scaffolding by endophilin, actin polymerization, and dynamin-mediated scission (Boucrot et al., 2015; Renard et al., 2015) (Fig. 1B, step 3). Multiple ligand-stimulated GPCRs can internalize via the FEME route through direct interaction between proline-rich motifs in their ICL3 and the SH3 domain of endophilins (Tang et al., 1999; Boucrot et al., 2015), but it is not yet understood how receptor stimulation triggers FEME (Fig. 1B, step 2).
Endocytosis via Caveolae.
Caveolae are invaginations of the plasma membrane, enriched for cholesterol and sphingomyelin (Ortegren et al., 2004). Their formation relies on the coordinated membrane-deforming activity of caveolin, cavin, and pacsin (also named syndapin) proteins (Ludwig et al., 2013) (Fig. 1C). Caveolins are cholesterol-binding proteins integrated in the inner leaflet of the plasma membrane that form 12- to 16-meric complexes (Ariotti et al., 2015). Mature caveolae are formed upon phosphatidylserine- and PI(4,5)P2-mediated association of coat-forming trimeric cavin complexes (Kovtun et al., 2014), FES/CIP4 homology–BAR domain–containing pacsin proteins (Hansen et al., 2011) and the EH domain–containing 2 protein (Yeow et al., 2017) with these caveolin-rich domains (Fig. 1C, step 2). Pacsin furthermore interacts with dynamin via its SH3 domain, facilitating caveolae vesicle scission (Koch et al., 2011).
Several GPCRs have been reported to localize in caveolae, interact with caveolin, and/or internalize via caveolae-mediated endocytosis (Chini and Parenti, 2004). GPCRs can directly bind to caveolin through φXφXXXXφ or φXXXXφXXφ motifs (X = any amino acid; φ = Phe, Trp, or Tyr) (Couet et al., 1997) (Fig. 1C, step 1a). Interaction with caveolin, however, does not necessarily dictate receptor endocytosis via caveolae, as caveolin can also function as chaperone during receptor transport to the cell surface or facilitate caveolae localization without triggering receptor internalization (Chini and Parenti, 2004). Also, Gαq subunits, but not Gβγ or other Gα proteins, can interact with caveolin, which facilitates association between receptors and caveolae (Sengupta et al., 2008; Calizo and Scarlata, 2012) (Fig. 1C, step 1b).
In contrast to clathrin-coated pits, the density of caveolae differs between tissues and cell types: whereas caveolae can be undetectable in some cell types, they can occupy up to 50% of the plasma membrane surface in others (Thorn et al., 2003; Zhuang et al., 2011). It is unclear whether differences in caveolae abundance affect endocytosis of receptors via this pathway. Furthermore, even though caveolae can bud from the plasma membrane and translocate to early endosomes (Hayer et al., 2010), caveolae are now primarily viewed as membrane structures with functions different from endocytosis, including membrane tension buffer and specialized lipid rafts important for signaling (Sinha et al., 2011; Shvets et al., 2015). Altogether, the molecular mechanisms driving GPCR internalization via caveolae are still poorly understood, and it is unknown how agonist stimulation triggers caveolae-mediated endocytosis of GPCRs.
Arrestin-Independent Agonist-Induced Endocytosis
Despite the well characterized role of arrestins in GPCR endocytosis, including inhibiting GPCR/G protein coupling and initiating internalization (Kang et al., 2013; Tian et al., 2014), there is now a considerable number of examples of arrestin-independent GPCR internalization upon agonist stimulation (Table 1). In several cases the mechanism has been investigated further, and alternative mediators of endocytosis have been identified, such as caveolae, endophilin, GRKs, clathrin, and clathrin adaptors.
GPCRs that have been shown to undergo arrestin-independent agonist-induced or constitutive endocytosis
Clathrin- and AP-2–Dependent Pathway.
In addition to the role of AP-2 in arrestin-mediated endocytosis, the μ2 adaptin subunit of AP-2 can directly interact with polyarginine motifs (Diviani et al., 2003), dileucine motifs ([D/E]XXXL[L/I], X = any amino acid) (Pandey, 2010), or tyrosine motifs (YXXΦ, Φ = bulky hydrophobic amino acid) (Ohno et al., 1995) in the intracellular loops or C-tail of GPCRs, through which it might facilitate clathrin-mediated endocytosis independently of arrestins.
The C-tail of proteinase-activated receptor (PAR) 1 contains a YXXL µ2 adaptin binding motif (Paing et al., 2004). Similar to the role of arrestins for other receptors, AP-2 interacts directly with PAR1 and is required for its constitutive and agonist-induced internalization through a clathrin- and dynamin-dependent pathway (Paing et al., 2006). Arrestins play a critical role in PAR1 desensitization to uncouple G protein signaling but are not essential for PAR1 internalization (Paing et al., 2002). Native receptors are found in two distinct pools: one in the cell membrane and one in an intracellular compartment (Shapiro et al., 1996). In the absence of agonist, the native receptor cycles between the cell membrane and the intracellular pool. Instead, internalized, activated PAR1 is sorted from endosomes to lysosomes, where it is rapidly degraded (Trejo et al., 1998). Studies using a combination of microscopy, mutant receptors, and pharmacological and genetic inhibitors showed that the two processes are dependent on distinct mechanisms. Constitutive internalization is effected upon mutation of the PAR1 tyrosine motif (Y383A/L386A) and by depletion of AP-2 using small interfering RNA (siRNA), indicating the importance of AP-2 in the process (Paing et al., 2006). On the other hand, agonist-induced internalization is only partially inhibited in AP-2–depleted cells and is reliant on additional sequences in the C-tail, suggesting the involvement of other clathrin adaptors (Paing et al., 2004, 2006; Trejo et al., 2000). Trejo and colleagues proposed that epsin-1 is the other key clathrin adaptor protein for active PAR1 internalization (Chen et al., 2011). Epsin-1–mediated endocytosis requires ubiquitination of PAR1, and the ubiquitin-interacting motifs of epsin-1 are crucial for this pathway. In cells depleted of epsin-1 and/or AP-2 by siRNA, activated PAR1 internalization was impaired, further confirming that both adaptor proteins are required for agonist-induced internalization.
Several other receptors that internalize through a partially or completely arrestin-independent mechanism also contain tyrosine motifs. PAR4 has a YXXL motif in ICL3, and a Y263A/L268A double mutation in the AP-2 binding motif disrupted the ability of the receptor to internalize (Smith et al., 2016). Similarly, depletion of AP-2 and clathrin by siRNA inhibited receptor internalization, thus confirming an AP-2– and clathrin-dependent mechanism. The IP prostanoid receptor has a YXXL motif in ICL2 (Smyth et al., 2000). Microscopy studies showed that the active receptor was present in clathrin-coated vesicles. This process is repressed by dominant negative dynamin (K44A), but it is arrestin- and GRK-independent and is not affected by PKC-mediated receptor phosphorylation. In contrast, the α1B-adrenoceptor contains several YXXΦ and dileucine motifs (Diviani et al., 2003), but AP-2 has not been shown to interact with these motifs. Instead, AP-2 binds to a stretch of eight arginine residues on the receptor C-tail. Arrestins contribute partially to α1B-adrenoceptor endocytosis, demonstrating that multiple pathways can regulate internalization of an individual receptor.
GRK-Dependent Pathway.
GRKs are serine/threonine kinases that phosphorylate active GPCRs, thus facilitating arrestin binding to the receptor and inhibiting G protein interactions (Benovic et al., 1986, (Bouvier et al., 1988); Komolov and Benovic, 2018). Seven mammalian GRKs (GRK1–7) that regulate GPCRs have been identified: GRK1 and GRK7 are expressed exclusively in the retina; GRK4 is only found in significant amounts in the testes, whereas GRK2, -3, -5 and -6 are universally expressed (Komolov and Benovic, 2018). In addition to their role in arrestin binding, there are several reports of an internalization pathway that is GRK phosphorylation-dependent but arrestin-independent.
Upon agonist stimulation, the BLT1 leukotriene receptor internalizes through a GRK2- and dynamin-dependent mechanism without involving arrestins (Chen et al., 2004b). Agonist stimulation of the receptor does not cause arrestin redistribution within the cell, and the receptor does not associate with arrestins. Moreover, receptor internalization is not affected by overexpression of wild-type and dominant negative arrestin-2 (V53D). The receptor undergoes endocytosis in rat basophilic leukemia (RBL)-2H3 cells that express high levels of endogenous GRK2, but not in human embryonic kidney 293 (HEK293) or COS-7 cells that express 5–10-fold less GRK2 than RBL-2H3 cells. Additionally, the process is blocked by coexpression of a catalytically inactive GRK2 mutant (K220R) and enhanced when wild-type GRK2 is overexpressed, which indicates that it is phosphorylation-dependent. A BLT1 receptor mutant with truncated C-tail lost the ability to internalize and associate with GRK2 and dominant negative dynamin (K44A) inhibited BLT1 endocytosis. However, the mechanism of how GRK2-mediated phosphorylation can trigger arrestin-independent endocytosis remains to be clarified.
Formylpeptide receptor 1 (FPR1) has also been suggested to internalize via a GRK phosphorylation-dependent and arrestin-independent pathway (Prossnitz et al., 1995; Hsu et al., 1997; Vines et al., 2003). A phosphorylation-deficient mutant of FPR1 where the serine and threonine residues in the C terminus are converted to alanine and glycine residues was unable to desensitize and internalize, thus indicating that phosphorylation is required for the processes (Hsu et al., 1997). GRK2 and to a lesser degree GRK3 were shown to be the kinases responsible for phosphorylating the FPR1 C-tail (Prossnitz et al., 1995). Although arrestins are colocalized with FPR1 in membranes and endosomes during receptor internalization (Bennett et al., 2000), they are not involved in internalization as determined by the use of dominant negative arrestin-2 (arrestin-2319−418) and mouse embryonic fibroblasts (MEFs) from arrestin-2/3 knockout mice (Gilbert et al., 2001; Vines et al., 2003). Nonetheless, arrestins have been suggested to play a role in FPR1 recycling to the plasma membrane because in arrestin-2/3 knockout MEFs the receptor accumulated in the perinuclear endosome compartment instead of recycling through an unknown mechanism (Vines et al., 2003). FPR1 internalization is furthermore insensitive to dominant negative mutants of dynamin (K44A) and clathrin (hub region, competes for binding to clathrin light chain) (Gilbert et al., 2001), which suggests that it is internalized through a different mechanism than the BLT1 receptor.
FEME Pathway.
β1- and α2A-adrenoceptors, D3 and D4 dopamine receptors, and the M4 muscarinic receptor have been demonstrated to internalize through the FEME pathway (Boucrot et al., 2015) (Fig. 1B). Agonist-induced internalization of these receptors was strongly reduced upon siRNA knockdown of endophilin-A1–3, but not with knockdown of clathrin or AP-2, thus indicating that it is an endocytic route that is independent of clathrin. Correspondingly, the process is not affected by overexpression or siRNA depletion of arrestin-2/3. Dynamin is identified as the main driver of the endophilin-mediated fission because dominant negative dynamin (K44A and K65A) and several small molecule dynamin inhibitors negatively affected the formation of endophilin buds. Using pharmacological inhibitors, this pathway was further shown to depend on cholesterol, actin, Rho GTPase, phosphatidylinositol 3-kinase, and the serine/threonine protein kinase PAK1 (Boucrot et al., 2015).
Caveolae Pathway.
Using arrestin-2/3 knockout HEK293 cells, the glucagon-like peptide (GLP)-1 receptor has been shown to internalize independently of arrestins (Jones et al., 2018). Studies applying confocal microscopy showed that the GFP-tagged receptor localizes in membrane lipid rafts and caveolae (Fig. 1C). As the receptor contains a classic caveolin-1 binding motif (EGVYLYTLLAFSVF) in ICL2, the receptor could interact directly with caveolin-1 and be endocytosed via caveolae. This is further evidenced using dominant negative mutants of caveolin-1 (P132L) and dynamin (K44A) that inhibit GLP-1 receptor endocytosis (Syme et al., 2006). The GLP-2 receptor within the same receptor family can most likely also be desensitized, internalized, and recycled independently of arrestins, since these processes were inert to receptor C-tail truncation (Estall et al., 2004, 2005). GLP-2 receptor endocytosis is inhibited by cholesterol sequestration with filipin or cholesterol depletion with methyl-β-cyclodextrin, thus suggesting that the receptor internalizes via a lipid-raft–mediated pathway. After endocytosis, the GLP-2 receptor colocalizes with caveolin-1 in early endosomes and perinuclear recycling compartments (Estall et al., 2004). However, unlike the GLP-1 receptor, the GLP-2 receptor lacks the classic caveolin-1 binding motif and is internalized independently of dynamin, thus suggesting different internalization pathways for the two receptors.
The ETA and ETB endothelin receptors are able to recruit arrestins but unable to promote association with AP-2 upon agonist stimulation, as shown using bioluminescence resonance energy transfer (BRET)–based assays for arrestin-2 and -3/β2 adaptin interactions (Hamdan et al., 2007). Furthermore, internalization of the ETA receptor is not blocked by the arrestin/β2 adaptin inhibitor barbadin (Beautrait et al., 2017). However, knockdown of arrestin-2/3 using siRNA showed partial inhibition of ETA and ETB receptor endocytosis (Hamdan et al., 2007). Thus, this indicates that these receptors internalize partially through a mechanism that involves a direct interaction between arrestin and clathrin and partially through an arrestin- and AP-2–independent pathway. In fact, around a third of all clathrin-coated pits have been found not to contain AP-2 (Pascolutti et al., 2019), which suggests that AP-2–independent clathrin-mediated endocytosis could be more common than previously anticipated. The ETA receptor undergoes caveolae-mediated internalization in HEK293 cells (Okamoto et al., 2000), which could account for the arrestin-independent component.
β2-adrenoceptors and angiotensin (AT)1A angiotensin receptors are shown to internalize via two distinct pathways, clathrin-mediated endocytosis and the caveolae endocytic route (Guo et al., 2015). Using specific inhibitors for clathrin (dominant negative epsin204–458) and caveolae (methyl-β-cyclodextrin) in cells depleted of clathrin and caveolin-1 by small hairpin RNA, the authors confirmed that these are different pathways. Moreover, clathrin-mediated endocytosis is mediated by GRKs, but caveolar endocytosis is not dependent on GRKs. The AT1A receptor also showed cell type–specific internalization. In HEK293 and COS-7 cells, receptor internalization was not mediated by dynamin or arrestins, but in CHO cells, it was abolished by hypertonic sucrose, dominant negative arrestins (arrestin-2-V53D and arrestin-21−349) and dominant negative dynamin K44A, thereby indicating that arrestins are involved in clathrin-mediated AT1A internalization in this cell line (Zhang et al., 1996; Oakley et al., 2000; Gáborik et al., 2001).
Arrestin-Independent Constitutive Endocytosis
GPCRs can also undergo endocytosis in the absence of agonist stimulation. In contrast to agonist-induced endocytosis, the mechanisms and functions of constitutive endocytosis are less well understood. Similar to active receptor endocytosis, there are examples of GPCRs that are able to internalize constitutively without the need for arrestins (Table 1).
Major Histocompatibility Complex Class I Pathway.
β2-adrenoceptor and M3 muscarinic receptors internalize without agonist stimulation and colocalize with major histocompatibility complex class I (MHC-I) on peripheral endosomal structures. MHC-I marks a clathrin-independent endocytic pathway. Their constitutive internalization is also not inhibited by dominant negative dynamin (K44A) and only slightly affected by siRNA depletion of clathrin, suggesting that dynamin and clathrin are not required. Upon agonist stimulation, these receptors switch to a clathrin-dependent trafficking pathway (Scarselli and Donaldson, 2009). The clathrin-independent endocytosis pathway used by MHC-I is believed to be independent of arrestins, but it remains to be confirmed if arrestins play a role in the constitutive internalization of the β2-adrenoceptor and the M3 receptor.
It is well established that the metabotropic glutamate (mGlu) receptors are unable to recruit arrestins (Pin and Bettler, 2016). In the absence of ligand, the mGlu7 receptor colocalizes with internalized MHC-I in endosomes (Lavezzari and Roche, 2007). It was found to traffic there via an ADP-ribosylation factor 6–positive endosomal pathway that is not regulated by clathrin. The mGlu5 receptor also internalizes through a clathrin-independent pathway in the absence of receptor activation (Fourgeaud et al., 2003); however, it contains caveolin-1 binding motifs in ICL1 and ICL3 and colocalizes with caveolin-1 in hippocampal neurons, and constitutive mGlu5 receptor internalization is inhibited by nystatin-mediated sequestration of cholesterol (Francesconi et al., 2009), thus suggesting that mGlu5 is constitutively internalized via caveolae.
Other Pathways.
Other GPCRs also internalize constitutively without the need for arrestins, but their exact mechanisms are not defined. Using ELISA and confocal microscopy, the orphan adhesion receptor ADGRA3 (previously called GPR125) was found to undergo rapid constitutive internalization in an arrestin-independent, but clathrin-dependent manner (Spiess et al., 2019). The internalized receptor colocalized with transferrin receptor 1 in early endosomes. Chemokine receptors CXCR4 and XCR1 and the viral GPCR US28 also show constitutive activity and internalization in the absence of arrestins (Fraile-Ramos et al., 2003; Bauer et al., 2019; Spiess et al., 2019). Like the β2-adrenoceptor and the M3 receptor, constitutive and agonist-induced internalization of CXCR4 occurs through distinct pathways. Constitutive internalization of the receptor is dependent on PKC and dynamin but seems to be independent of arrestins, as internalization was not affected by deleting potential arrestin-2/3 binding sites from the C-tail of CXCR4. However, activated CXCR4 internalizes through the arrestin-mediated pathway (Signoret et al., 1997, 1998). Similarly, the calcium-sensing (CaS) receptor undergoes constitutive internalization through a pathway that is partially arrestin-independent, whereas agonist-mediated internalization was found to be arrestin-2/3-dependent (Mos et al., 2019). Altogether, several GPCRs have been reported to internalize constitutively in an arrestin-independent manner. Although the molecular mechanisms at play during arrestin-independent internalization are often not investigated, these studies show that ligand-stimulated and constitutive receptor endocytosis can differ in terms of arrestin dependency.
Arrestin Activation without Triggering Internalization.
As described above, FPR1 and PAR1 recruit arrestins upon activation but internalize independently of arrestins. Instead, arrestins are proposed to regulate PAR1 desensitization (Paing et al., 2002) and FPR1 recycling (Vines et al., 2003). Most of the receptors that have been reported to internalize independently of arrestins (Table 1) have in fact been shown to recruit arrestin-2 and/or -3 to the plasma membrane in a recent systematic study using a receptor-independent enhanced bystander BRET assay (Avet C et al., preprint, DOI: https://doi.org/10.1101/2020.04.20.052027). These inconsistencies could be a result of different cellular backgrounds or experimental conditions, such as the need for overexpression of arrestin in most assays measuring arrestin recruitment. However, it is intriguing to consider the possibility that GPCRs can interact with arrestin without triggering internalization and the potential functional consequences of such an interaction.
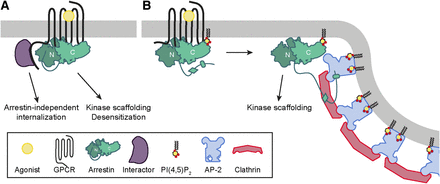
Would it be possible for a receptor to interact with arrestin without exposing the AP-2 and clathrin binding motifs in the arrestin C-tail that is bound to the arrestin N-domain in inactive arrestin (Fig. 2A)? Several studies have used intramolecular biosensors to show that arrestins can adopt multiple active conformations with distinct functions (“active arrestin” is defined in the following as a conformation that involves a major conformational change from the inactive state, which exposes functionalities, such as protein and lipid binding sites that were inaccessible in the inactive state). This was first shown with arrestin-2 or -3 biosensors with BRET donors and acceptors fused to each end of arrestin (Shukla et al., 2008; Nobles et al., 2011; Zimmerman et al., 2012). Whereas endogenous agonists for AT1A angiotensin and parathyroid (PTH) 1 parathyroid hormone receptors [angiotensin II and parathyroid hormone (PTH)-1-34, respectively] increased the intramolecular arrestin BRET signal, the arrestin-biased ligands [Sar1, Ile4, Ile8]-angiotensin II and [D-Trp12, Tyr34]-PTH-(7–34) decreased the BRET signal, thus indicating different arrestin conformations (Shukla et al., 2008). Similarly, when introducing point mutations in the transmembrane segment of the AT1A receptor or the β2-adrenoceptor that interfere with G protein coupling, thus generating an arrestin-biased receptor, the intramolecular BRET signal upon stimulation with angiotensin II or isoproterenol changed from increasing to decreasing. The intramolecular biosensor approach was later extended by inserting short (six amino acids) binding motifs for fluorescein arsenical hairpin (FlAsH) in different places in arrestin-3 and monitoring either Förster resonance energy transfer with a C-terminal cyan fluorescent protein (Nuber et al., 2016) or BRET with an N-terminal Renilla luciferase (Lee et al., 2016). These biosensors confirmed the existence of receptor and ligand specific arrestin conformations. The conformational signatures of arrestin furthermore correlated with receptor trafficking and arrestin-dependent extracellular signal-regulated kinase (ERK) 1/2 phosphorylation patterns (Lee et al., 2016). Although it is tempting to speculate that some of these biosensors could provide evidence for arrestin activation without arrestin C-tail displacement, such inferences are notoriously difficult to make for biosensors. Recently, the biosensor experiments were corroborated by a study using an intracellularly expressed antibody fragment (intrabody) specific for active arrestin-2 (Baidya et al., 2020). The binding of the intrabody to arrestin-2 was triggered by ligand stimulation of the V2 vasopressin receptor, but not by the B2 bradykinin receptor, although both recruited arrestin-2 to a similar extent. It is thus possible that some GPCRs can stabilize an arrestin conformation that does not lead to internalization but supports other arrestin functions, although such a conformation has not yet been demonstrated directly.
Nonconventional receptor-arrestin interactions that do not lead to arrestin-dependent internalization. (A) Hypothetical interaction between arrestin and the 7TM core of a receptor that stabilizes an intermediate state of arrestin without displacement of the arrestin C-tail from the arrestin N-domain. The clathrin and AP-2 interaction motifs in the arrestin C-tail are still masked, but arrestin blocks G protein activation and could potentially potentiate signaling by scaffolding kinases. This model allows simultaneous interaction of the receptor C-tail with a hypothetical protein (depicted as interactor) that mediates receptor internalization. (B) Catalytic activation of arrestin. Arrestin is activated by a transient interaction with the receptor 7TM core and is stabilized at the membrane in this conformation by binding of PI(4,5)P2. Activated arrestins accumulate in clathrin-coated structures where they can scaffold kinases to enhance signaling.
A possible mechanism for stabilizing different arrestin conformations could be by arrestin interactions with discrete receptor sites. The prevailing model for arrestin activation was for many years a multistep model where arrestin first recognizes either the phosphorylated C-tail of the receptor or the seven transmembrane (7TM) core of an activated receptor, which constitutes a low-affinity precomplex. Arrestin can then proceed to engage the other binding site if it is present, thus forming the high-affinity complex that is required for arrestin activation (Gurevich and Benovic, 1993). Later studies have, however, revealed that engagement of only one of these sites is sufficient for activation of arrestin (Richardson et al., 2003; Kumari et al., 2016, 2017; Cahill et al., 2017; Latorraca et al., 2018). Several studies have looked at the correlation between the functional consequences of receptor-arrestin interactions and whether arrestin is binding to the receptor 7TM core, the phosphorylated C-tail, or both. For receptors with a C-tail that forms stable complexes with arrestins, such as the V2 receptor, the core interaction was shown to be important for rapid desensitization of G protein signaling, but the interaction between the phosphorylated receptor C-tail and arrestin was sufficient to mediate internalization and arrestin-dependent ERK phosphorylation (Kumari et al., 2016, 2017; Cahill et al., 2017). This is consistent with PAR1 where G protein desensitization by arrestin is independent of phosphorylation (Chen et al., 2004a). Conversely, a truncation mutant of the substance P receptor that removes all serine and threonine residues from the C-tail desensitized and internalized like the full-length receptor (Richardson et al., 2003). Similarly, a phosphorylation-deficient mutant of the BLT1 leukotriene receptor was still able to recruit arrestin and internalize, although with a delay compared with the wild-type receptor (Jala et al., 2005). Thus, only desensitization of G protein signaling seems to be specifically linked to interaction with the receptor 7TM core, whereas internalization and enhancement of signaling can be mediated by interaction with either of the two binding sites but seem to be linked as long as arrestin is associated with the receptor.
Displacement of the arrestin C-tail has been shown to enable arrestin to spontaneously undergo conformational changes to a presumably active conformation where the C-domain of arrestin is twisted 20° relative to the N-domain (Latorraca et al., 2018). This is supported by the fact that the naturally occurring p44 splice variant of arrestin-1 that lacks the C-tail has been crystallized in both active and inactive conformations (Granzin et al., 2012; Kim et al., 2013). Allosteric coupling between the two events would suggest that the converse is also true, i.e., that the conformational rearrangement of the N- and C-domains that normally occurs upon receptor binding would lead to C-tail displacement and subsequently receptor endocytosis. However, if multiple active arrestin conformations exist, as suggested by intramolecular biosensor experiments, we speculate that some of these conformations might not displace the arrestin C-tail. Indeed, differential phosphorylation of the receptor C-tail has been suggested to induce different arrestin conformations by selectively interacting with a distinct subset of the key elements that stabilize the inactive state of arrestin (Sente et al., 2018). Interestingly, proximal phosphorylation appears to release the finger loop that is important for interacting with the receptor 7TM core, but possibly not the three-element interaction between α-helix 1 and β-strand 1 of the arrestin N-domain and the arrestin C-tail.
None of the structures of receptor-arrestin complexes published so far has retained autoinhibitory binding of the arrestin C-tail. However, these structures used either phosphorylated receptor C-tails that are known to bind strongly to arrestin (Shukla et al., 2014; Lee et al., 2020; Staus et al., 2020), truncated the arrestin C-tail (Huang et al., 2020; Staus et al., 2020), or destabilized the arrestin C-tail binding through mutations in the arrestin C-tail (Kang et al., 2015; Yin et al., 2019), thus making it unlikely or impossible to preserve autoinhibitory arrestin C-tail binding. Structures of receptors without a phosphorylated C-tail in complex with full-length arrestin are eagerly awaited to shed light on whether it is possible to retain binding of the arrestin C-tail when bound to a receptor.
An alternative model of arrestin activation termed catalytic activation has been described, where arrestin interacts transiently with the receptor 7TM core but remains bound to the membrane in an active conformation stabilized by phosphoinositide binding (Fig. 2B) (Eichel et al., 2016, 2018; Nuber et al., 2016). When arrestin dissociates from the receptor, it can no longer drive receptor internalization, but it can still traffic to clathrin-coated structures and mediate ERK1/2 phosphorylation from there. This catalytic activation mechanism was found for several receptors that are known to interact transiently with arrestin: the β1- and β2-adrenoceptors, the D2 dopamine receptor, and the µ and κ opioid receptors (Eichel et al., 2016, 2018).
In conclusion, arrestins can block G protein activation without triggering endocytosis by binding to the 7TM core of stimulated receptors in a mechanism that most likely does not involve major conformational changes in arrestin. There is evidence from intramolecular arrestin biosensor experiments that a given receptor-ligand combination could stabilize a specific arrestin conformation, but the role of arrestins in FPR1 recycling after arrestin-independent endocytosis remains the only functional or structural evidence of a receptor-arrestin complex that mediates arrestin functions requiring arrestin activation without also driving receptor internalization (Vines et al., 2003). Catalytic activation of arrestin does, however, provide such a mechanism, and it would be interesting to determine if receptors that internalize in an arrestin-independent way accumulate active arrestins at clathrin-coated structures.
Methods for Measuring Endocytosis
It has for many years been technically challenging to measure receptor endocytosis with the same level of sensitivity and robustness as receptor signaling, which made detailed analysis of endocytic pathways challenging. However, there are now sensitive and robust internalization assays that can be combined with imaging for a complete picture of receptor endocytosis.
Imaging.
The most direct method to track subcellular distribution of GPCRs is by fluorescence microscopy (Hislop and von Zastrow, 2011; Foster and Bräuner-Osborne, 2018). The receptor usually has to be genetically tagged with an epitope or fluorescent protein or undergo enzyme-directed covalent modification to allow for examination and localization using fluorescence microscopy (Daunt et al., 1997; Hislop and von Zastrow, 2011; Cahill et al., 2017). The method is flexible, widely available, and applicable to most GPCRs. Furthermore, it can be used in vivo, by knocking in the tagged receptor in animals, allowing for real-time imaging of receptor trafficking in e.g., neurons (Ehrlich et al., 2019). A potential drawback is that recombinantly expressed and modified receptors may not exactly mimic the properties of native receptors.
BRET and Time-Resolved Förster Resonance Energy Transfer.
BRET and time-resolved Förster resonance energy transfer (TR-FRET) internalization assays have higher throughput than imaging assays but lack the spatial resolution. BRET trafficking assays require heterologous coexpression of fusion proteins with a luciferase variant that can catalyze the generation of donor bioluminescence and a fluorescent protein acceptor to measure proximity between GPCRs and compartment markers in real time (Hamdan et al., 2005, 2006; Pfleger et al., 2007). The acceptor can be anchored to distinct cell compartments by genetically fusing it to targeting sequences for the plasma membrane (e.g., CAAX), the endoplasmic reticulum (e.g., PTP1B), or different stages of endosomes (e.g., Rab5, Rab7, or Rab11) (Pfleger and Eidne, 2006; Lan et al., 2012; (Szakadáti et al., 2015) Namkung et al., 2016; Cahill et al., 2017). When GPCRs relocate to these compartments, bystander BRET will be generated that allows for the study of the complete trafficking cycle (Balla et al., 2012; Cao et al., 2019). However, the specificity of the bystander BRET assay should be taken into consideration, as it relies on the assumption of a homogenous cell population with similar levels of fusion protein expression and requires fusion of a luciferase on the intracellular side of receptors, which could interfere with trafficking.
The TR-FRET internalization assay can also effectively assess the time course of receptor internalization. It requires fusion of an N-terminal SNAP-tag to receptors, which is less likely to interfere with trafficking than an intracellular tag and is applicable to a wide range of GPCRs (Roed et al., 2014; Jacobsen et al., 2017; Foster et al., 2019). The SNAP-tagged receptors are covalently labeled with a cell-impermeant terbium cryptate substrate (SNAP-Lumi4-Tb) that acts as energy donor and ensures that only receptors that are at the cell surface during labeling are tracked. Lanthanide complexes, such as terbium cryptate, have millisecond lifetimes, which makes it possible to introduce a delay between excitation and recording of emission, eliminating short-lived fluorescence background from, for example, cellular autofluorescence (Levoye et al., 2015). After washing to remove excess donor substrate, cells are incubated with a cell-impermeant energy acceptor (e.g., fluorescein-O′-acetic acid). For receptors at the cell surface, energy is transferred from the donor to the acceptor upon excitation of the donor, thus resulting in a low donor-acceptor ratio. Receptor internalization (constitutive or agonist-induced) causes an increased donor-acceptor distance that is incompatible with energy transfer, which leads to an increased donor/acceptor ratio (Foster and Bräuner-Osborne, 2018). The TR-FRET internalization assay uses a synthetic fluorophore in solution as the acceptor, which limits the assay to measuring receptor endocytosis.
ELISA, Flow Cytometry, Immunoblotting, and Radioligand Binding.
Other high-throughput GPCR endocytosis assays include biochemical measurements of receptors with N-terminal epitope tags, such as hemagglutinin and Flag tags (Kang et al., 2013; Foster and Bräuner-Osborne, 2018). Examples include ELISA, flow cytometry, and immunoblotting to quantify loss of cell surface GPCR and determine the rate of internalization upon agonist stimulation (Okamoto et al., 2000). For receptors with readily available radioligands, different protocols of binding experiments can be performed to compare cell surface with total receptor density (Heilker, 2007). However, this is limited to receptors with specific high-affinity radiolabeled ligands. Also, these assays are only able to measure net changes in receptor number, which is why it can be challenging to effectively distinguish between the effects of receptor biosynthesis and degradation, in contrast to the BRET assays that can track receptor quantity changes in different cellular compartments (Hislop and von Zastrow, 2011).
Tools for Testing Arrestin Involvement in Endocytosis.
A variety of methods have been used to determine whether receptor endocytosis depends on arrestins, to further examine the molecular machinery involved, and to identify the distinct endocytic routes. Here we present a critical overview of some of the most commonly used tools, including their advantages and possible pitfalls, with focus on probing the involvement of arrestins.
Genetic Approaches.
To determine the involvement of arrestins in GPCR desensitization, internalization, and downregulation, a wide range of pharmacological and genetic tools have been developed. Arguably, the most effective method to study their impact on receptor endocytosis is to genetically deplete endogenous arrestins from the cellular background. Different methods have been used to successfully achieve this, including the use of RNA interference to knockdown arrestins (Ahn et al., 2003; Wei et al., 2003; Shenoy et al., 2006; O’Hayre et al., 2017; Luttrell et al., 2018). However, full knockdown of expression is rarely achieved with this method, which complicates analysis of the results. In contrast, MEFs prepared from arrestin-2/3 knockout mice can be used to perform experiments in the complete absence of arrestins. These MEFs have been used to study the contribution of arrestin to the endocytosis of several GPCRs, including the β2-adrenoceptor, the FPR1 formylpeptide receptor, the PAR1 and PAR4 proteinase-activated receptors, and the viral chemokine receptor US28 (Kohout et al., 2001; Paing et al., 2002; Fraile-Ramos et al., 2003; Vines et al., 2003; Smith et al., 2016). More recently, advancement of the CRISPR/CRISPR-associated protein genome editing technology has made specific gene targeting widely available. This led to the generation of a wide range of HEK293 cell lines where genes encoding different proteins have been knocked out, including GPCRs, G proteins, GRKs, and arrestins (Milligan and Inoue, 2018; Møller et al., 2020). The arrestin-2/3 knockout cells have been used to express and study GPCRs such as the β2-adrenoceptor and the CaS, gastric inhibitory polypeptide, µ-opioid and V2 vasopressin receptors and to assess the role of arrestins in receptor endocytosis as well as their function in cell signaling (Gabe et al., 2018; Luttrell et al., 2018; Mos et al., 2019; Møller et al., 2020). The cell lines were validated to be completely deprived of arrestins, and, compared with knockout animals, they are less likely to have altered levels of other network components to compensate for the loss of the deleted genes, as arrestin-2/3 knockout cells did not have significantly altered expression of G proteins (Alvarez-Curto et al., 2016).
Dominant Negative Protein Mutants.
Another commonly used group of tools to study GPCR endocytosis is dominant negative mutants. These mutants are able to compete with the wild-type protein for some of the same binding partners but are unable to perform certain key functions. They will effectively inhibit the action of the wild-type protein when expressed in excess (Sheppard, 1994). For arrestins, there are several dominant negative mutants that effectively inhibit agonist-promoted endocytosis by interfering with the ability of arrestins to bind to GPCRs (arrestin-2-V53D, arrestin-3-V54D) (Ferguson et al., 1996), clathrin (arrestin-2-ΔLIELD), or β2 adaptin (arrestin-2-F391A) (Kim and Benovic, 2002) or by competing with clathrin binding (arrestin-2319−418 and arrestin-3284−409) (Krupnick et al., 1997; Orsini and Benovic, 1998).
Pharmacological Inhibitors.
Finally, pharmacological inhibitors can be used to decipher the contribution of their target protein in the receptor endocytosis process. Barbadin is an inhibitor that selectively binds to AP-2 and disrupts the interaction between arrestins and the β2 adaptin subunit of AP-2 (Beautrait et al., 2017). Other pharmacological inhibitors include pitstop2 that blocks both clathrin-dependent and -independent endocytosis (Dutta et al., 2012), dynasore and dyngo-4a that inhibit dynamin-dependent pathways (Macia et al., 2006; Hill et al., 2009; McCluskey et al., 2013), nystatin and filipin that disrupt caveolae-mediated internalization, and chlorpromazine that blocks internalization via clathrin-coated pits (Okamoto et al., 2000). However, experimental results based on these inhibitors need to be interpreted with care, as the selectivity of these compounds may not be as defined as previously thought (Ivanov, 2008; Park et al., 2013; Guo et al., 2015). Pitstop2, for instance, is still being advertised as a clathrin-selective inhibitor, even though equipotent inhibition of clathrin-independent endocytosis was reported long ago (Dutta et al., 2012).
Conclusions
GPCR endocytosis is often assumed to be an arrestin-mediated process. Here we have presented more than 30 examples of arrestin-independent agonist-induced or constitutive endocytosis (Table 1), which show that GPCR endocytosis is a more diverse process than initially expected. However, for most of the receptors that are shown to internalize independently of arrestins, there is little known about the alternative pathway. The absence of clear discriminators between the pathways has resulted in a lack of tools to specifically study them. Further complicating matters, agonists that selectively activate one endocytosis pathway have been described, for example, for the D3 dopamine receptor (Xu et al., 2019). Moreover, receptors display cell type–specific endocytosis, indicating that cellular background and molecular makeup of the environment can drive distinct internalization pathways. It is thus necessary for the field to apply the recent technological developments that are outlined in this review, such as high-throughput internalization assays and CRISPR/CRISPR-associated protein genome editing, to delineate the arrestin-independent internalization pathways and thereby expand our understanding of GPCR regulation.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Moo, van Senten, Bräuner-Osborne, Møller.
Footnotes
- Received October 27, 2020.
- Accepted January 7, 2021.
This project received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreements no. 797497 (T.C.M.) and no. 846827 (E.V.M.). The authors declare no conflicts of interest.
Abbreviations
- AP-2
- adaptor protein 2
- AT
- angiotensin
- BAR
- Bin/amphiphysin/Rvs
- BRET
- bioluminescence resonance energy transfer
- CaS
- calcium-sensing
- Cdc42
- cell division control protein 42 homolog
- C-domain
- C-terminal domain
- CIP4
- Cdc42-interacting protein 4
- C-tail
- carboxy-terminal tail
- ERK
- extracellular signal-regulated kinase
- FEME
- fast endophilin-mediated endocytosis
- FlAsH fluorescein arsenical hairpin FPR1
- formylpeptide receptor 1
- GLP
- glucagon-like peptide
- GPCR
- G protein–coupled receptor
- GRK
- GPCR kinase
- HEK293
- human embryonic kidney 293
- ICL
- intracellular loop
- MEF
- mouse embryonic fibroblast
- MHC-I
- major histocompatibility complex class I
- mGlu
- metabotropic glutamate
- N-domain
- N-terminal domain
- PAR
- proteinase-activated receptor
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PKC
- protein kinase C
- PTH
- parathyroid hormone
- RBL
- rat basophilic leukemia
- SH3
- Src homology 3
- siRNA
- small interfering RNA
- 7TM
- seven transmembrane
- TR-FRET
- time-resolved Förster resonance energy transfer
- Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics